Precious metal nanoparticle-modified porous carrier catalytic material and preparation method thereof
A porous carrier and catalytic material technology, applied in chemical instruments and methods, catalyst carriers, physical/chemical process catalysts, etc., can solve problems such as dropping, aging of nanoparticles, and poor performance of repeated use, and achieve convenient recycling and high efficiency The effect of high, few steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
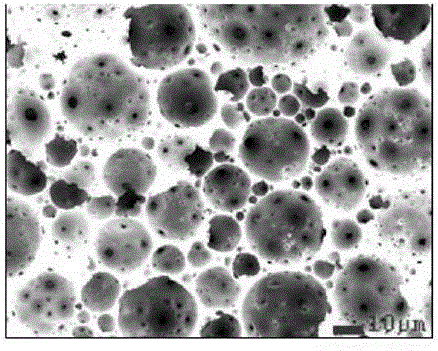
[0045] PEI (1 gram, Aldrich company product, molecular weight 10000) and polystyrene (PS, 6 grams, molecular weight 7500) reaction synthesis amphiphile (synthesis can refer to open patent: 201410663000.x or according to literature Wan DC, Yuan JJ, Pu HT. Macromolecules 2009, 42, 1533). PEIPS was dissolved in chloroform (50 mL). Chlorauric acid (0.25 g) was then dissolved in a small amount of water (5 ml) and added to the above chloroform solution with stirring. Due to the acid-base complexation and automatic reduction of PEI and chloroauric acid, the unique red color of gold nanometers will be formed soon after the oil-water two-phase is mixed and stirred. Add inorganic desiccant sodium sulfate to dry the water, separate the liquid, and further centrifuge the obtained chloroform system or remove the chloroform by rotary evaporation, and collect the solid. The formation of gold nanometers was confirmed by ultraviolet / visible light spectroscopy, transmission electron microscop...
Embodiment 2
[0049] Prescription is substantially the same as embodiment 1 but preparation method is different. The oil phase is composed of styrene St, polyethylene glycol dimethacrylate (PEGDMA), toluene, AIBN initiator and PEIPS, and the specific volume composition is: St+PEGDMA:60% (St / PEGDMA=8:2), Toluene: 30%, PEIPS: 10%; AIBN: 1% of the mass of the oil phase. In addition, water equivalent to 8 times the volume of the oil phase (constituted by phosphate buffer solution (0.01M) solution, pH7.4) and chloroauric acid (formulation is the same as in Example 1) is dissolved in the water sample, and added dropwise to the Stir in the oil phase. Stirring was continued for several minutes after dropping, and the formed emulsion was placed in a convection heater at 70° C. for 12 hours. Wash with ethanol to obtain light brown rigid solid. The apparent density determined by the Archimedes method is 0.15 grams per milliliter. The scanning electron microscope energy spectrum characterization sh...
Embodiment 3
[0051] With embodiment 2, but replace chloroauric acid with silver nitrate and operate equally. The obtained porous carrier catalytic material is brown to light black. The scanning electron microscope energy spectrum characterization shows that the silver element is uniformly distributed on the surface and cannot be detected at all in the cross section.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com