A kind of goat pox, sheep pox bivalent cellular attenuated vaccine and its preparation method and application
A technology of attenuated vaccine and sheep pox virus, which is applied to sheep pox bivalent cell attenuated vaccine and preparation thereof, and the field of goat pox, can solve the problems of high cost of vaccine production, transportation and storage, heat-labile freeze-drying protective agent, etc. The formula ingredients and preparation method are simple, and the immune protection power and heat resistance are guaranteed.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Isolation and weakening of embodiment 1 goat pox virus (Goatpox virus HN-XY 2010)
[0036] 1.1 Isolation and identification of goat pox virus (Goatpox virus HN-XY 2010)
[0037] The inventors of the present application carried out virus isolation and case replication on goat pox-positive disease materials collected from Xinyang, Henan in 2010, and finally obtained a virus strain, which was identified by virus physicochemical characteristics and specific for goat pox virus P32 gene PCR detection and sequencing proved that the virus strain was goat pox virus virus (named Gotepox Virus HN-XY10 strain, referred to as GTPV / HN / 2010 strain).
[0038] 1.2 Attenuation of goat pox virus (Goatpox virus HN-XY2010 strain) seedling strain and establishment of virus seed batches
[0039] The GTPV / HN / 2010 strain virus was subcultured to 16 passages with the primary cells of sheep testis, and the GTPV virulence lgTCID 50 ≥3.5, choose 9-day-old to 11-day-old SPF chicken embryos, remove...
Embodiment 2
[0047] Isolation and attenuation of embodiment 2 sheep pox virus (Sheeppox Virus GS-WW 2010 strain)
[0048] 2.1 Isolation and identification of sheep pox virus (Sheeppox Virus GS-WW 2010 strain)
[0049] The inventor of the present application isolated the virus from sheep pox-positive disease materials collected in Wuwei, Gansu in 2010, replicated the case, and finally obtained a strain of the virus. PCR detection and sequencing proved that the virus strain was a sheep pox virus virus named (Sheeppox Virus GS-WW 2010, referred to as SPPV / GS / 2010 strain).
[0050] 2.2 Attenuation of sheep pox virus (Sheeppox Virus GS-WW 2010 strain) seedling strain and establishment of virus seed batches
[0051] The SPPV / GS / 2010 strain virus was subcultured to 17 passages with the primary cells of sheep testis, and the GTPV virulence lgTCID 50 ≥3.5, choose 9-day-old to 11-day-old SPF chicken embryos, remove the head, limbs, and viscera of the chicken embryo under aseptic conditions, cut th...
Embodiment 3
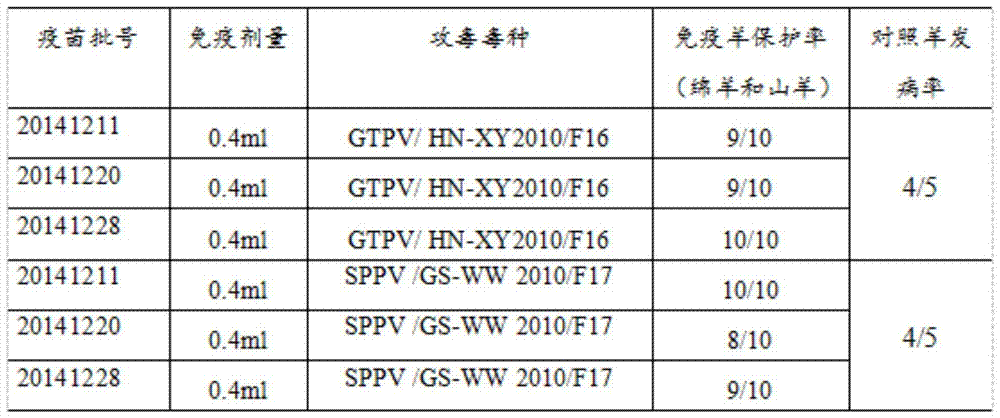
[0059] Example 3 Laboratory Trial Production of Goat Pox and Sheep Pox Virus Duplex Cell Attenuated Vaccine
[0060] 3.1 Preparation of goat pox and sheep pox virus attenuated antigens
[0061] Select neonatal goat testicular primary cells (LT) that grow well and cover a single layer, pour off the nutrient solution, and inoculate goatpox virus cell passage attenuated vaccine strain Goatpox Virus HN-XY2010F43 (CCTCC NO : V201503) and sheep pox virus cell passaging attenuated vaccine strain Sheeppox Virus GS-WW 2010F44 (CCTCC NO: V201504), add DMEM maintenance solution after 10 minutes of adsorption, adjust the pH to 7.0, add penicillin and streptomycin 100 units / ml, at 37°C, 5% CO 2 Cultured in a concentration incubator. Cultivate for 48 hours, harvest when the cytopathic pathology (CPE) reaches 80%, put it in a -20°C refrigerator and freeze and thaw twice, then carry out sterility testing, safety testing, mycoplasma testing, and exogenous virus testing to meet the requireme...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com