A kind of Japanese encephalitis/yellow fever chimeric virus and its preparation method and application
A chimeric virus, yellow fever technology, applied in the directions of botanical equipment and methods, biochemical equipment and methods, viruses, etc., to achieve the effects of good safety, reduced toxicity, and low toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Embodiment 1 Construction of chimeric virus of the present invention
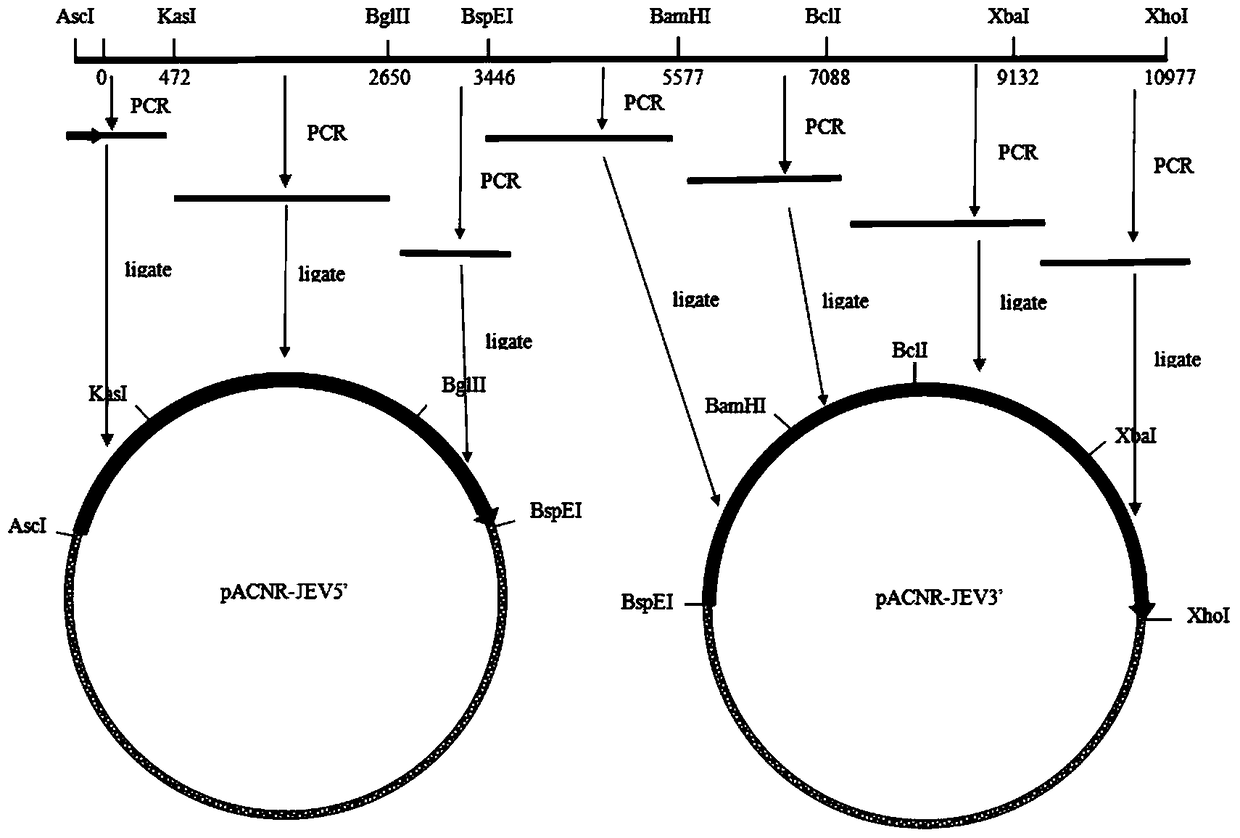
[0032] 1. Construction and identification of full-length infectious full-length cDNA clone containing JEV vaccine strain SA14-14-2 and recovery of recombinant virus
[0033] 1. Modification of multiple cloning sites of low-copy plasmid pACNR
[0034] Dissolve Oligo-1 (5'-CGCGCCATTAGGCGCCTTATAGATCTAATGTCCGGATTATGGATCCTGATTGATCATTATTTCTAGAATTAC-3', respectively, the underlines are KasI, BglII, BspEI, BamHI, BclI, XbaI recognition sites) and Oligo-2 (5'-TCGAGTAATTCTAGAATAATGATCAATCAGGATCCATAATCCGGAAGGC-AGATA', respectively 1 After the complementary double strands are formed, the 5' and 3' ends form AscI and Xhol enzyme-digested sticky ends respectively, both of which are prepared by Shanghai Yingwei Jieji Biotechnology Co., Ltd.) in 100 μl sterilized double-distilled water, mix 10 μl each, and heat Cool naturally after reaching 100°C, take 2 μl and connect with pACNR double-digested with AscI and Xhol ...
Embodiment 2
[0094] The preparation of embodiment 2 vaccines of the present invention
[0095] The chimeric virus Chimeri-JYF prepared in Example 1 was inoculated into primary hamster kidney cells (PHK cells) according to 0.001-0.1 M.O.I, and after being adsorbed at 36°C±1°C for 1h, added with a final concentration of 0.1% human serum white The protein MEM maintenance solution was cultured at 36°C±1°C for 2-5 days. After the cells showed obvious CPE (cytopathic changes), the supernatant was harvested and filtered to become the vaccine stock solution.
[0096] The vaccine of the present invention is obtained by adding pharmaceutically acceptable adjuvants or auxiliary components to the vaccine stock solution.
[0097] The beneficial effect of the present invention is illustrated below in the mode of experimental example:
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com