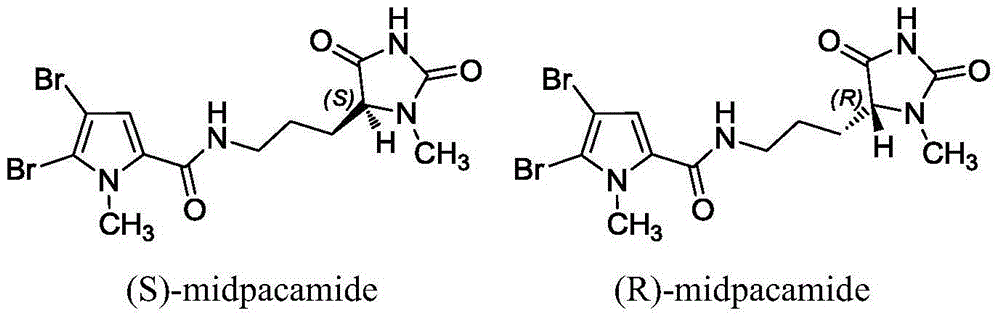
Synthetic method for chiral marine natural product with high optical activity
A technology of optical activity and synthesis method, applied in the fields of organic chemistry, bulk chemical production, organic chemistry, etc., can solve the problems of complex process, difficult mass production, cumbersome route steps, etc., and achieves broad application prospects and mild reaction conditions. , the effect of simple synthesis steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
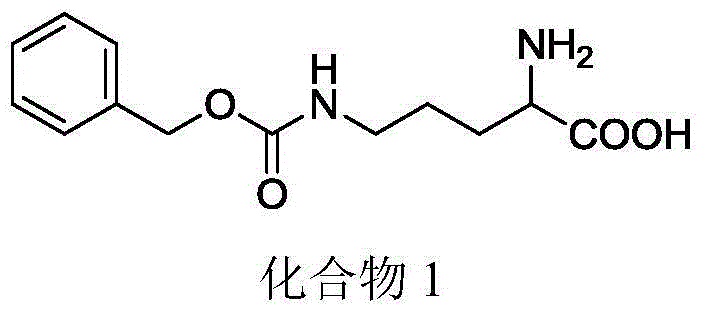
[0055] Example 1: Preparation of 2-amino-5-(((benzyloxy)carbonyl)amino)pentanoic acid
[0056] Dissolve 7.5g of L-ornithine hydrochloride in 89ml of 0.5mol / L sodium hydroxide solution, add 5.55g of anhydrous copper sulfate, react for 15min, then add 6.15g of anhydrous potassium carbonate and 8.2ml of benzyl chloroformate , reacted overnight, filtered and washed to obtain a blue solid, then added saturated disodium ethylenediaminetetraacetic acid solution, first heated to reflux for 2 hours, then overnight at room temperature, filtered, washed and dried to obtain a white solid, with a yield of 83%.
Embodiment 2
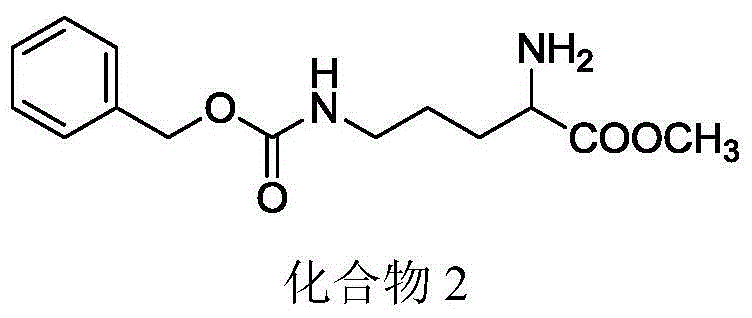
[0057] Example 2: Preparation of 2-amino-5-(((benzyloxy)carbonyl)amino)methyl pentanoate
[0058] Add 8.5ml of acetyl chloride dropwise to methanol under ice-bath condition, and rise to room temperature to react for 10min. 10.6 g of compound 1 was added to the reaction solution, refluxed for 5 hours, and the solvent was evaporated, ethyl acetate was added, after washing and drying, the solvent was evaporated to obtain a light yellow solid with a yield of 85%. 1 H-NMR (300MHz, CDCl 3 ):δ1.36-1.61(m,2H),1.74-1.82(m,2H),2.97-3.03(q,2H),3.74(s,3H),4.05(s,1H),δ5.01(s ,2H),δ7.28-7.40(m,6H),δ8.51(s,3H); 13 C-NMR (100MHz, CDCl 3 ): δ25.3, 27.4, 40.1, 52.9, 53.3, 66.5, 128.0, 128.5, 128.6, 136.7, 156.8, 170.2.
Embodiment 3
[0059] Example 3: Preparation of methyl 5-(((benzyloxy)carbonyl)amino)-2-(3-methylureido)valerate
[0060] Dissolve 11.2 g of compound 2 in a mixed solution of dichloromethane and saturated sodium bicarbonate under ice bath conditions, add 5.29 g of triphosgene, react for 15 minutes and extract quickly, and dry the organic phase to obtain a dichloromethane solution of isocyanate. Under an ice bath, the dichloromethane solution of isocyanate was dropped into the dichloromethane solution containing methylamine and triethylamine, and reacted at room temperature for 1 hour after the dropping was completed. After distilling off the solvent, ethyl acetate was added, the organic phase was washed and dried, and the solvent was distilled off. Recrystallization gave a white solid with a yield of 78% and a melting point of 114-117°C. 1 H-NMR (300MHz, DMSO-d 6 ):δ1.38-1.49(m,2H),δ1.52-1.69(m,2H),δ2.51-2.54(d,3H),2.95-3.01(q,2H),δ3.60(s, 3H),δ4.07-4.15(q,1H),δ5.00(s,2H),δ5.82(d,1H),δ6.2...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| optical purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com