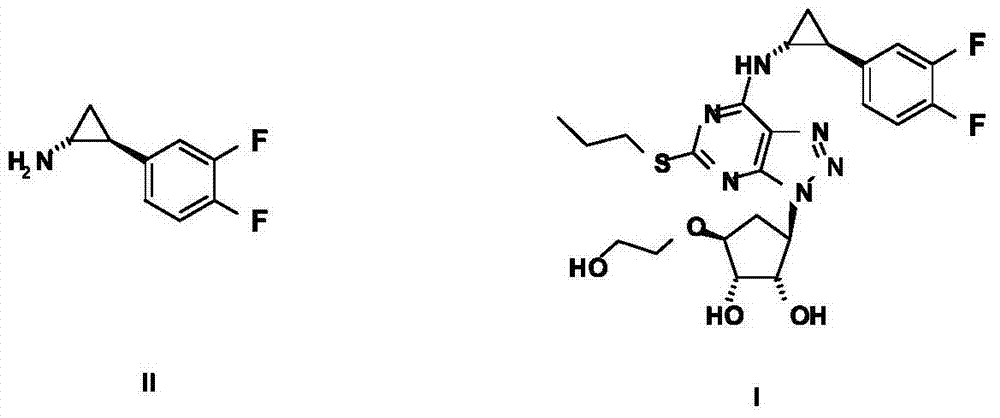
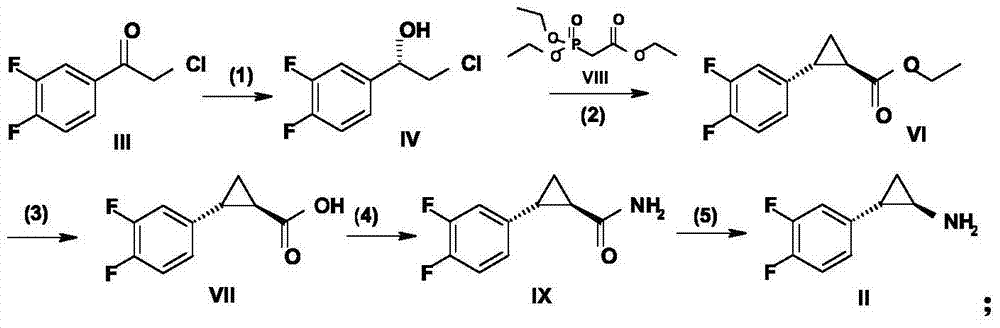
Preparation method of ticagrelor intermediate
A technology for ticagrelor and intermediates, which is applied in the field of preparation of small molecule anticoagulant ticagrelor intermediates, can solve problems such as danger, environmental unfriendliness, and environmental hazards, so as to improve operational safety and improve Generating efficient, environmentally friendly results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] Embodiment 1: The specific preparation method of formula IV compound (S)-2-chloro-1-(3,4-difluorophenyl)ethanol:
[0054] A solution consisting of N,N-diethylaniline hydrochloride (18.0 g, 97 mmol) and 60 ml of dichloromethane was added dropwise at 0°C to sodium borohydride (7.2 g, 190 mmol) and In the suspension composed of 160 ml of ethylene glycol dimethyl ether, keep the reaction temperature at 20-30 degrees, and keep the temperature for 4 hours after dropping. A solution consisting of S-diphenylprolinol (2.7 g, 10.7 mmol) and 10 mL of dichloromethane was added. 2-Chloro-1-(3,4-difluorophenyl)ethanone (20.4 g, 107 mmol) was dissolved in 60 ml of dichloromethane and dropped into the above reaction system. After dripping, keep warm for 2 hours. 360 ml of 1N HCl was slowly added dropwise, the layers were separated, the water phase was removed, and the oil phase was spin-dried. Add 100 ml of ethyl acetate and wash three times with 1N HCl (100 ml each time). The oily...
Embodiment 2
[0060] Embodiment 2: The specific preparation method of formula IV compound (S)-2-chloro-1-(3,4-difluorophenyl)ethanol:
[0061] A solution consisting of N,N-diethylaniline hydrochloride (29.8 g, 160.5 mmol) and 150 ml of chloroform was added dropwise at 0°C to sodium borohydride (2.1 g, 54 mmol) and ethylene di In the suspension composed of 80 ml of alcohol dimethyl ether, keep the reaction temperature at 20-30 degrees, and keep the temperature for 24 hours after dropping. A solution consisting of S-diphenylprolinol (1.35 g, 5.3 mmol) and 10 mL of dichloromethane was added. 2-Chloro-1-(3,4-difluorophenyl)ethanone (20.4 g, 107 mmol) was dissolved in 60 ml of chloroform and dropped into the above reaction system. After dripping, keep warm for 2 hours. 90 ml of 1N HCl was slowly added dropwise, the layers were separated, and the aqueous phase was removed. The oil phase was washed three times with 1N HCl (100 ml each time), dried with anhydrous sodium sulfate, and spin-dried t...
Embodiment 3
[0063] Embodiment 3: the concrete preparation method of formula IV compound:
[0064] A solution consisting of N,N-diethylaniline hydrochloride (19.8 g, 107 mmol) and 198 ml of dichloromethane was added dropwise at 0°C to sodium borohydride (4.05 g, 107 mmol) and In the suspension composed of 150 ml of ethylene glycol dimethyl ether, keep the reaction temperature at 20-30 degrees, and keep the temperature for 10 hours after dropping. A solution of S-diphenylprolinol (1.35 g, 5.3 mmol) in 6 ml of dichloromethane was added and stirred for an additional hour. 2-Chloro-1-(3,4-difluorophenyl)ethanone (20.4 g, 107 mmol) was dissolved in 60 ml of dichloromethane and dropped into the above reaction system. After dripping, keep warm for 2 hours. 180 ml of 1N HCl was slowly added dropwise, and the aqueous phase was separated and removed. Add 100 ml of ethyl acetate and wash three times with 1N HCl (100 ml each time). The separated oily phase was dried with anhydrous sodium sulfate a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com