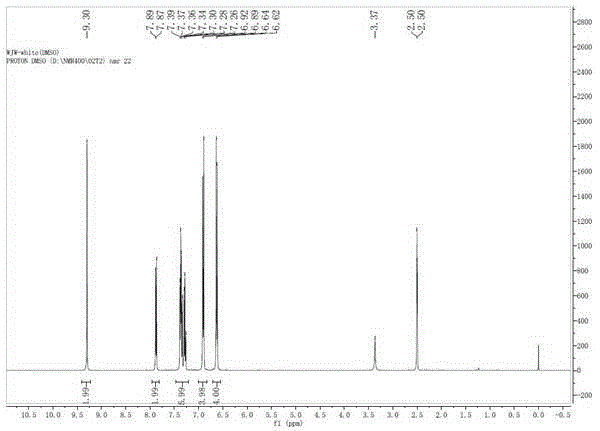
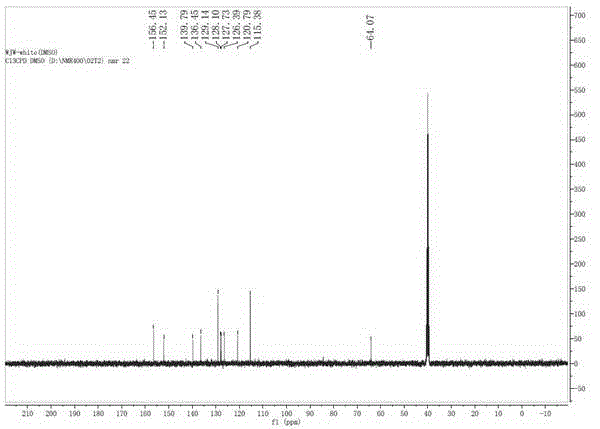
Green synthesis method of 9, 9-bis (4-hydroxy) fluorene
A synthesis method and phenol technology, which is applied in the field of synthesis of bisphenol fluorene, can solve the problems of difficult industrialization, non-environmental protection, and high phenol content, and achieve the effects of reducing phenol content, reducing environmental pressure, and high selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Add 10 grams of 9-fluorenone (0.055mol), 21 grams of phenol (0.22mol), and 16 grams of toluene to a 100ml flask equipped with a mechanical stirrer, a thermometer, and a condenser. Stir and place it in a water bath, then add 1.5 grams of Concentrated sulfuric acid, control the temperature at about 20 degrees, slowly add 0.37 g of 3-mercaptopropionic acid (0.0035 mol), after the addition, react at 20 ° C for 2 hours, slowly raise the temperature to 30 ° C and continue the reaction for 1 hour. After the system completely turned into a pure white slurry, liquid phase analysis was carried out, and the reaction of 9-fluorenone was complete, with a liquid phase content of 95% (excluding phenol). After adding 30 grams of toluene, cool down to room temperature and filter, and the filter cake is washed with a small amount of toluene. The filter cake was beaten in 5% sodium hydroxide solution with a mass ratio of 1:1, filtered, and dried to obtain 18.1 grams of pure white solid wi...
Embodiment 2
[0033] To a 100ml flask equipped with a mechanical stirrer, a thermometer, and a condenser, add 10 grams of 9-fluorenone (0.055mol), 31.5 grams of phenol (0.33mol), and 16 grams of dichloroethane in sequence, place them in a water bath with stirring, and add 2.5 grams of concentrated sulfuric acid, control the temperature at about 20°C, slowly add 0.37 grams of 3-mercaptopropionic acid (0.0035mol), after the addition, react at 20°C for 2 hours, slowly raise the temperature to 30°C and continue the reaction for 1 hour. After the system completely turned into a pure white slurry, liquid phase analysis was carried out, and the reaction of 9-fluorenone was complete, with a liquid phase content of 95% (excluding phenol). After adding 30 grams of dichloroethane, cool down to room temperature and filter, and the filter cake is washed with a small amount of dichloroethane. The filter cake was beaten in a 5% sodium hydroxide solution with a mass ratio of 1:1, filtered, and dried to obt...
example 3
[0036] Add 10 grams of 9-fluorenone (0.055mol), 21 grams of phenol (0.22mol), and 16 grams of toluene to a 100ml flask equipped with a mechanical stirrer, a thermometer, and a condenser. Stir and place it in a water bath, then add 2.5 grams of Concentrated sulfuric acid, control the temperature at about 20°C, slowly add 0.185 g of 3-mercaptopropionic acid (0.0018mol), after the addition, react at 20°C for 2 hours, slowly raise the temperature to 60°C and continue the reaction for 2 hours. After the system completely turned into a pure white slurry, liquid phase analysis was carried out, and the reaction of 9-fluorenone was complete, with a liquid phase content of 95% (excluding phenol). After adding 30 grams of toluene, cool down to room temperature and filter, and the filter cake is washed with a small amount of toluene. The filter cake was beaten in 5% sodium hydroxide solution with a mass ratio of 1:1, filtered, and dried to obtain 18.1 grams of pure white solid with a yiel...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com