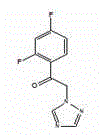
Pharmaceutical composition containing voriconazole
A technology of voriconazole and its composition, which is applied in the field of preparation of pharmaceutical composition and its tablet, can solve problems such as side effects, difference in dissolution rate, low bioavailability, etc., achieve good stability, complete dissolution, and simple production and operation Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
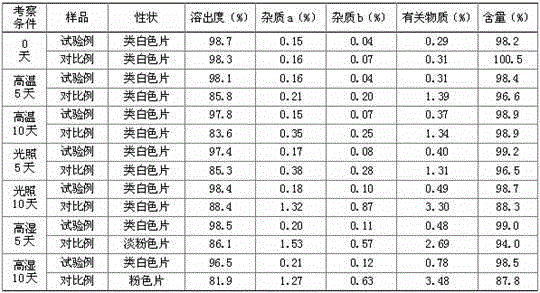
Examples
Embodiment 1
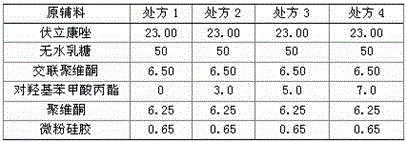
[0073] prescription
[0074] Voriconazole 10g
[0075] dry starch 4g
[0076] Micronized silica gel 1.8g
[0077] Mannitol 50g
[0078] Propylparaben 2g
[0079] Povidone 6g
[0080] Preparation
[0081] (1) After mixing 10g of voriconazole, 2g of propyl p-hydroxybenzoate, and 10g of mannitol, carry out microwave vacuum drying;
[0082] (2) The mixture obtained in step (1) is crushed, passed through an 80-mesh sieve, and set aside;
[0083] (3) Pass the remaining 40g of mannitol, 4g of dry starch, and 1.8g of micropowdered silica gel through an 80-mesh sieve, and set aside;
[0084] (4) Pour the mannitol, dry starch, and micropowdered silica gel sieved in step (3), and the mixture obtained in step (2) into a mixer for mixing;
[0085] (5) The mixture in step (4) is crushed at a low temperature and at a slow speed, and passed through a 80-mesh sieve;
[0086] (6) Add 6 g of povidone to the granules obtained in step (5) as a binder, mix, and press into tablets.
Embodiment 2
[0088] prescription
[0089] Voriconazole 15g
[0090] Sodium carboxymethyl starch 8g
[0093] Propylparaben 4.7g
[0094] Povidone 9.3g
[0095] Preparation
[0096] (1) After mixing 15g of voriconazole, 4.7g of propyl p-hydroxybenzoate, and 15g of anhydrous lactose, carry out microwave vacuum drying;
[0097] (2) The mixture obtained in step (1) is crushed, passed through an 80-mesh sieve, and set aside;
[0098] (3) Pass 30g of anhydrous lactose, 8g of sodium carboxymethyl starch, and 2.8g of talcum powder through an 80-mesh sieve respectively, and set aside;
[0099] (4) Pour the anhydrous lactose, sodium carboxymethyl starch, and talcum powder sieved in step (3), and the mixture obtained in step (2) into a mixer for mixing;
[0100] (5) The mixture in step (4) is crushed at a low temperature and at a slow speed, and passed through a 80-mesh sieve;
[0101] (6) Add 9.3 g of povidone to the granules obtained ...
Embodiment 3
[0103] prescription
[0104] Voriconazole 20g
[0105] Low-substituted hydroxypropyl cellulose 12g
[0106] Micronized silica gel 2.5g
[0108] Propylparaben 3.7g
[0109] Povidone 12g
[0110] Preparation
[0111] (1) After mixing 20g of voriconazole, 3.7g of propyl p-hydroxybenzoate and 10g of anhydrous lactose, carry out microwave vacuum drying;
[0112] (2) The mixture obtained in step (1) is crushed, passed through a 100-mesh sieve, and set aside;
[0113] (3) Pass 30g of anhydrous lactose, 12g of low-substituted hydroxypropyl cellulose, and 2.5g of micropowder silica gel through a 100-mesh sieve, and set aside;
[0114] (4) Pour the remaining amount of anhydrous lactose, low-substituted hydroxypropyl cellulose, micronized silica after sieving, and the mixture obtained in step (2) into a mixer for mixing;
[0115] (5) The mixture in step (4) is pulverized at a low temperature and slowly, and passed through a 100-mesh sieve;
[0116]...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com