Nitrogen-chlorine type chlorination agent
A chlorinated reagent, nitrogen-chlorine type technology, applied in the field of chlorinated reagent synthesis, can solve the problems of difficult storage or limitations of reaction substrates, inability to be widely used, severe reaction conditions, etc., and achieves a wide range of reaction substrates, The effect of good chlorination activity and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
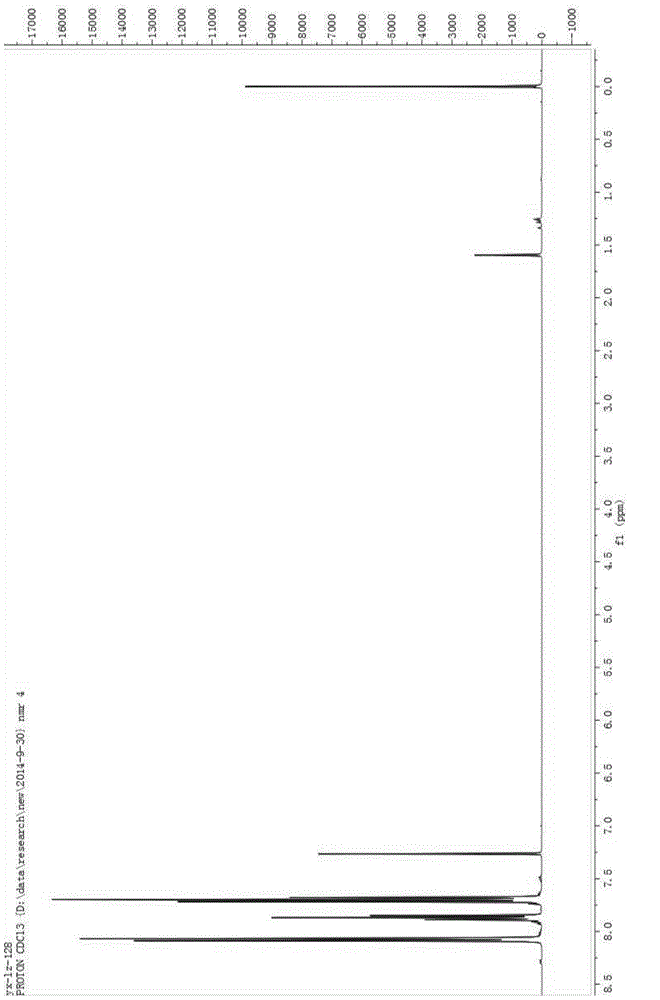
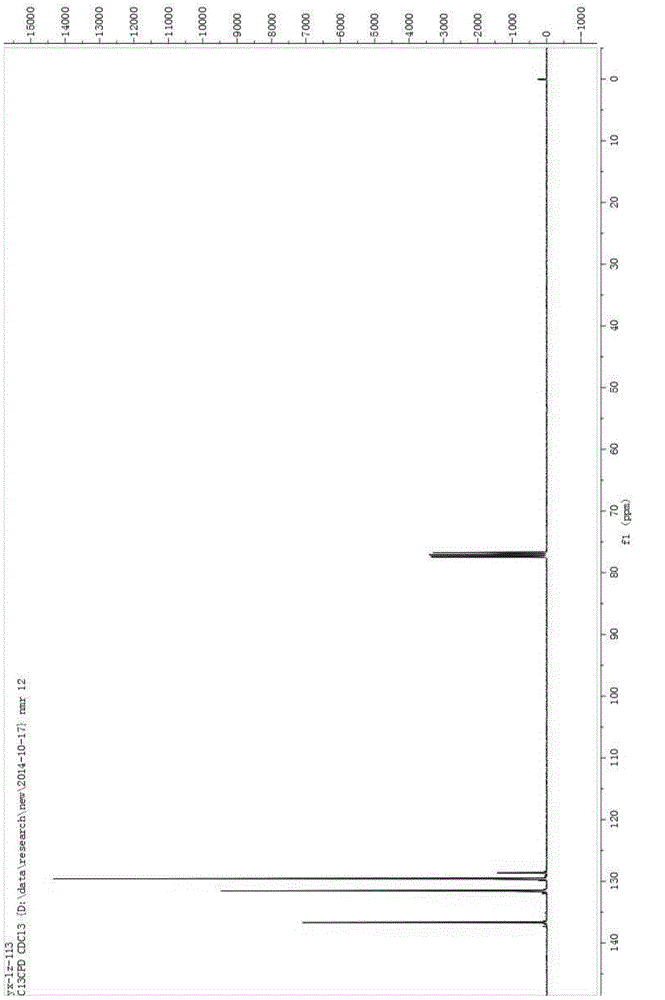
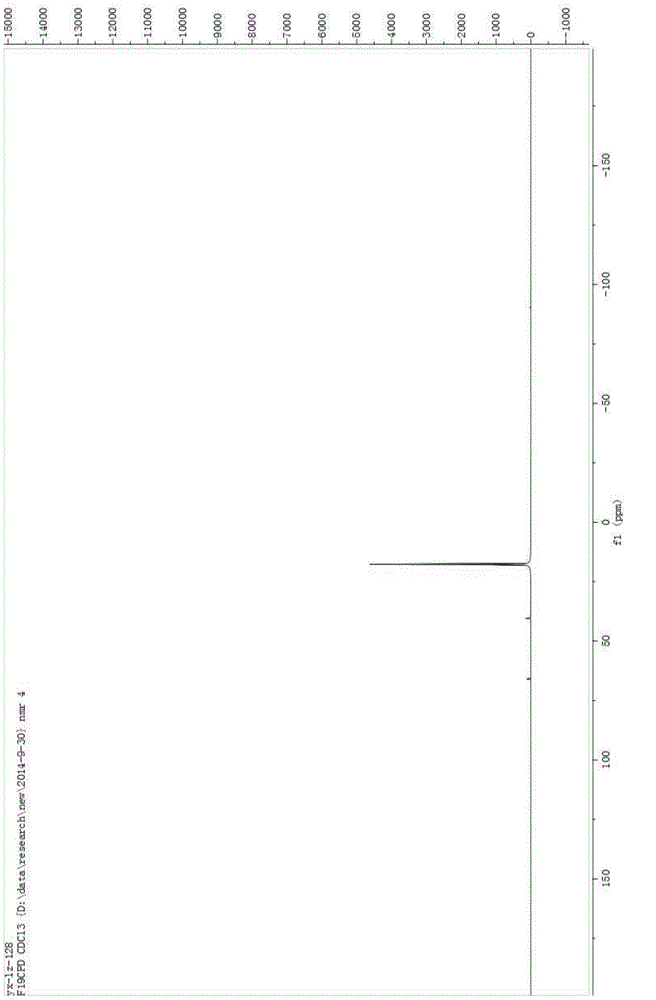
[0029] Add 100mL of acetonitrile, 5g of sodium hydroxide, and 10g of benzenesulfonamide into a 250mL three-neck flask, cool down to -10°C, pass in fluorine gas, and separate after the reaction is complete. The separation method is column chromatography. The chromatographic type is adsorption column chromatography, the column temperature is normal temperature (20-35°C), the adsorbent is silica gel, the eluent is petroleum ether and ethyl acetate, gradient elution, the column diameter height is 10:1, and the dry method is used. Column, dry loading, the elution rate is 1-2 drops per second. The eluent is PE:EA=10:1 (volume ratio), and the separated eluate is rotary evaporated to remove the solvent to obtain the product, which is a light yellow solid (10.2g); add the light yellow solid obtained in the previous step, 5g sodium hydroxide , Chlorine gas is passed through, and the reaction is completed and then separated, and the separation method is column chromatography. The chroma...
Embodiment 2
[0034] Add 5 mL of acetonitrile, 0.037 g of potassium carbonate, 0.04 g of the chlorination reagent, and 0.050 g of indole derivatives into a 25 mL one-necked flask, stir overnight at room temperature, and monitor the reaction by TLC. After the reaction was complete as monitored by TLC, it was separated by column chromatography. The chromatographic type is adsorption column chromatography, the chromatographic column temperature is normal temperature (20-35°C), the adsorbent is silica gel, gradient elution, the column diameter is 15:1, the dry method is packed, the dry method is added, and the elution speed is 1-2 drops per second. The eluent was PE:EA=10:1 (volume ratio), and the separated eluent was rotary evaporated to remove the solvent to obtain the chlorinated product (0.049 g, about 90% yield). The obtained chlorinated product was characterized by nuclear magnetic resonance and mass spectrometry, and the structure of the product was correct.
[0035] The reaction flow ...
Embodiment 3
[0038] Add 5 mL of acetonitrile, 0.041 g of potassium carbonate, 0.05 g of the chlorination reagent, and 0.050 g of the reaction substrate into a 25 mL single-necked flask, stir overnight at room temperature, and monitor the reaction by TLC. After the reaction was complete as monitored by TLC, it was separated by column chromatography. The chromatographic type is adsorption column chromatography, the chromatographic column temperature is normal temperature (20-35°C), the adsorbent is silica gel, gradient elution, the column diameter is 15:1, the dry method is packed, the dry method is added, and the elution speed is 1-2 drops per second. The eluent was PE:EA=15:1 (volume ratio), and the separated eluent was rotated to remove the solvent to obtain the chlorinated product (0.054 g, yield about 85-95%). The obtained chlorinated product was characterized by nuclear magnetic resonance and mass spectrometry, and the structure of the product was correct.
[0039] The reaction flow ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com