A clinical-grade serum-free medium for adherent culture of human neural stem cells
A serum-free medium, neural stem cell technology, applied in the field of stem cell culture, to achieve the effect of improving utilization, avoiding potential animal endotoxin virus infection, and increasing cell proliferation rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Example 1: Comparison of neural stem cell adherence and suspension culture to clinical transformation.
[0042] The key to the clinical application of neural stem cells lies in the large-scale preparation of stem cells and the stem cells used meet the requirements of clinical use. According to current literature reports, the neural stem cells in adherent culture proliferate faster and adherent cells are easier to prepare on a large scale.
[0043] The adherent cultured cells (P3) and suspension cultured cells (P3) were digested with Accutase enzyme, and the cells were collected and stored in liquid nitrogen at the same density with the same cryopreservation solution for three months. Resuscitated the cells for viable cell count and at the same density Inoculate the coated culture plate to observe the cell morphology. Results The survival rate of suspended cells after cryopreservation was only about 60%, while the survival rate of adherent cultured cells was over 90%. One d...
Embodiment 2
[0044] Example 2: Screening of ingredients in serum-free medium additives
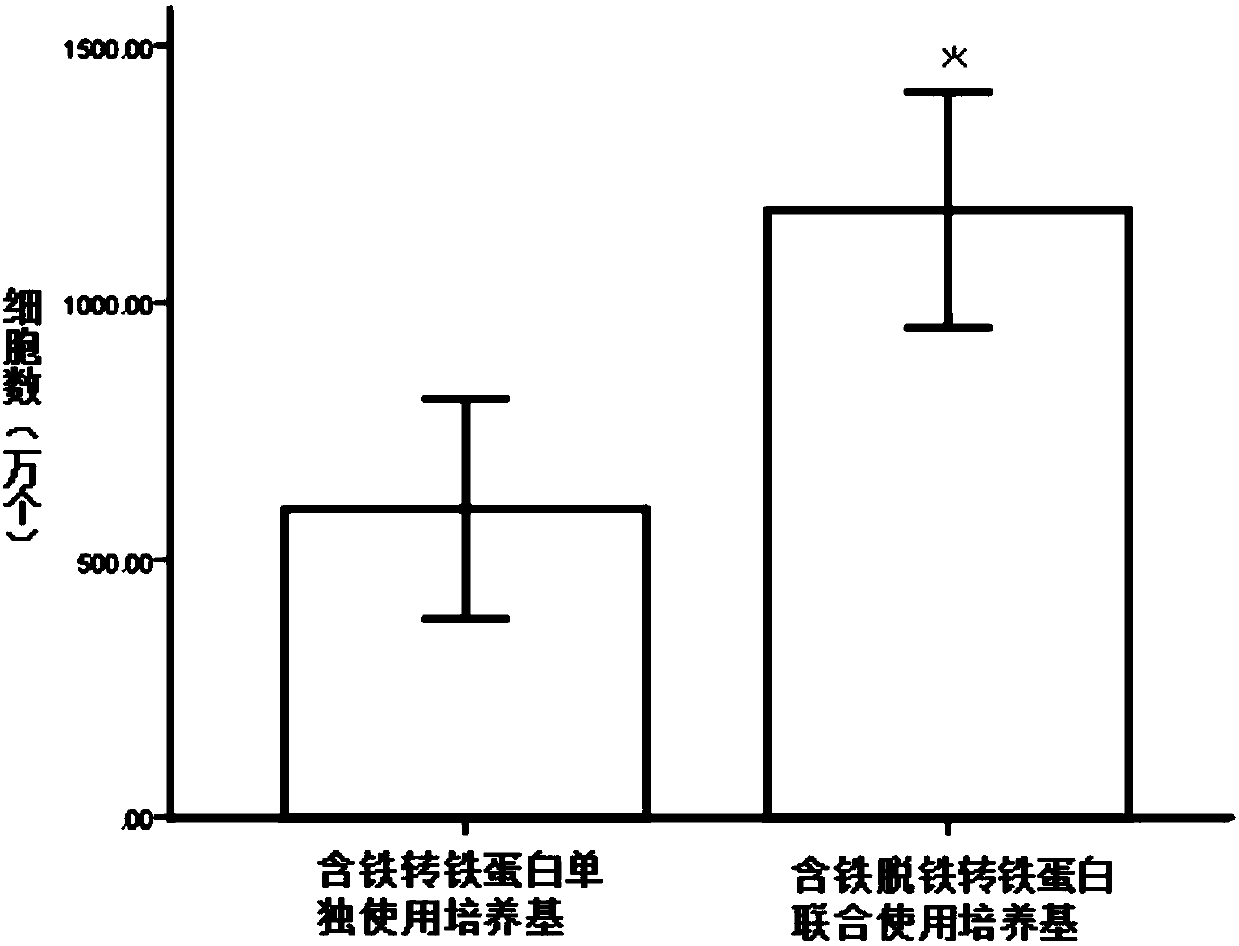
[0045] By consulting various materials, various additives that may be beneficial to cell proliferation were screened out including the following, insulin, transferrin, progesterone, putrescine, sodium selenite, apotransferrin, HSA, LIF, B-ME, VA, VC, Ve, Ve acetate, estradiol, thyroxine, ethanolamine, lipids, TGFβ, hydrocortisone, catalase, superoxide dismutase, glutathione , Triiodothyronine, L-carnitine, galactose, etc. Among them, the first five are N2 components. The present invention formulates them according to the concentration of each component in N2 as a basic addition, and then adds appropriate concentrations of other various cytokines, BFGF, and EGF to cultivate NSC from P0 to P3. Cell proliferation speed, cell morphology and cell proliferation ability after passage are used as evaluation indicators to comprehensively evaluate the effects of various factors on cell proliferation and differenti...
Embodiment 3
[0056] Example 3 The serum-free stem cell culture of the present invention that can be used for culturing human neural stem cells in the clinic has been tested for quality and all indicators are up to standard, as shown in Table 4:
[0057] Table 4: Test items and results of serum-free medium for neural stem cells of the present inventors
[0058]
[0059]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com