Reproducible catalyst for converting polycyclic aromatic hydrocarbon into monocyclic aromatic hydrocarbon and preparation method of reproducible catalyst
A technology for polycyclic aromatic hydrocarbons and monocyclic aromatic hydrocarbons, applied in the field of catalysts and their preparation, can solve the problems of low conversion depth of polycyclic aromatic hydrocarbons, poor catalyst regeneration performance, low yield of single-cyclic aromatic hydrocarbons, etc., and achieve maximum yield and selectivity The effect of chemicalization, good stability, and delaying the inactivation rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] On a dry basis, 25 g MOR type zeolite (commercially available ammonium type), 25 g ZSM-12 molecular sieve (commercially available hydrogen type) and 50 g γ-Al 2 o 3(Industrial product) Add to the mixer until homogeneous, then add 3 g of scallop powder, 5 g of HNO at a volume ratio of 1:1 to the mixture 3 solution and 40 g deionized water, and grind evenly to make a dough suitable for extrusion. It was extruded through a die in the shape of a slender cylinder (diameter 1.7 mm), dried at 120 °C and calcined at 550 °C for 4 h, and then cut into carrier particles (1.7 × 4.0 mm) with the same size. for Z1.
[0036] Prepare a solution of chloroplatinic acid and impregnate the carrier particles at 40°C. The amount of Pt contained in the impregnating solution is 0.2% of the weight of the carrier on a dry basis. After 8 hours of immersion and drying, it is calcined at 450°C for 3 hours to obtain a finished product. Catalyst A.
[0037]
Embodiment 2~10
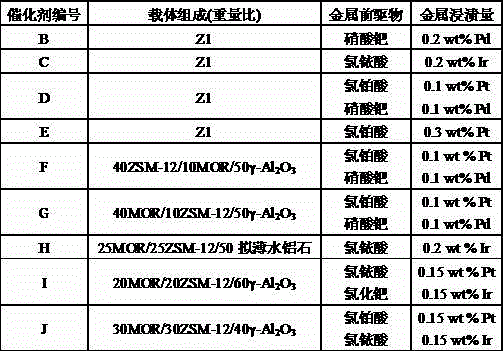
[0039] According to the preparation method and process provided in Example 1, a series of finished catalysts were prepared by changing the carrier composition and the type and content of metal precursors in the impregnation solution, as shown in Table 1.
[0040] Table 1
[0041]
Embodiment 11~19
[0043] Catalysts A~J prepared by Examples 1~10 are investigated on a supercritical fixed-bed reactor (maximum reaction pressure 10MPa, operating temperature is room temperature~600°C) and the polycyclic aromatic hydrocarbon mixture is converted into BTX (wherein B is benzene, T is toluene, X is the ability of monocyclic aromatic hydrocarbons such as xylene). Table 2 is the evaluation raw material composition. The catalyst is loaded with 5 g, at a reaction temperature of 350 ° C, a reaction pressure of 5.5 MPa, and a weight hourly space velocity of 2.0 hr -1 , Hydrogen / hydrocarbon molar ratio of 6.0 and contact with the catalyst, the product is recovered and component detection, the conversion results are shown in Table 3.
[0044]
[0045] Table 2
[0046] Raw material composition Non-Fang benzene toluene Ethylbenzene Xylene Decalin tetralin naphthalene Content (wt%) 0.02 24.54 24.61 5.00 0.02 14.81 15.25 15.75
[0047] table 3 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com