Medicinal ultrahigh-field nuclear-magnetism contrast agent and preparation method thereof
A nuclear magnetic contrast agent and ultra-high field technology, which can be used in pharmaceutical formulations, preparations for in vivo testing, emulsion delivery, etc., and can solve problems such as unfavorable ultra-high-field nuclear magnetic resonance imaging and unsuitability for ultra-high-field nuclear magnetic contrast agents, etc. Achieve the effect of promoting blood circulation performance, excellent imaging effect, and improving efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
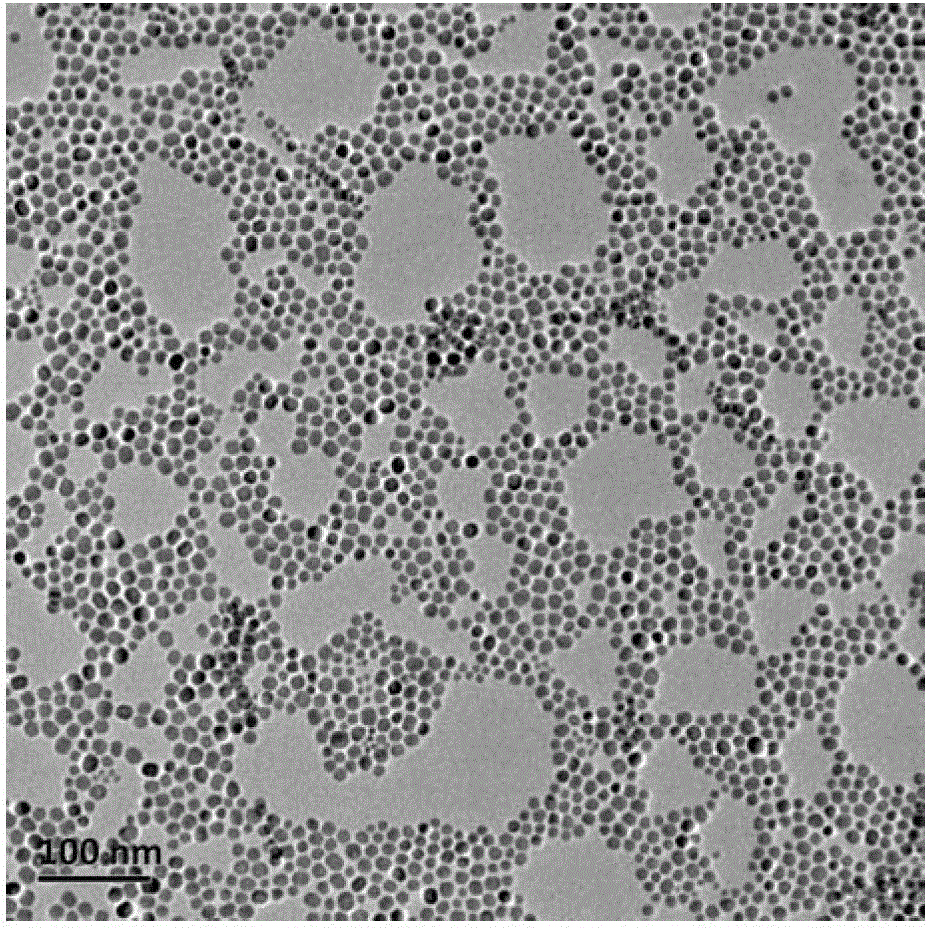
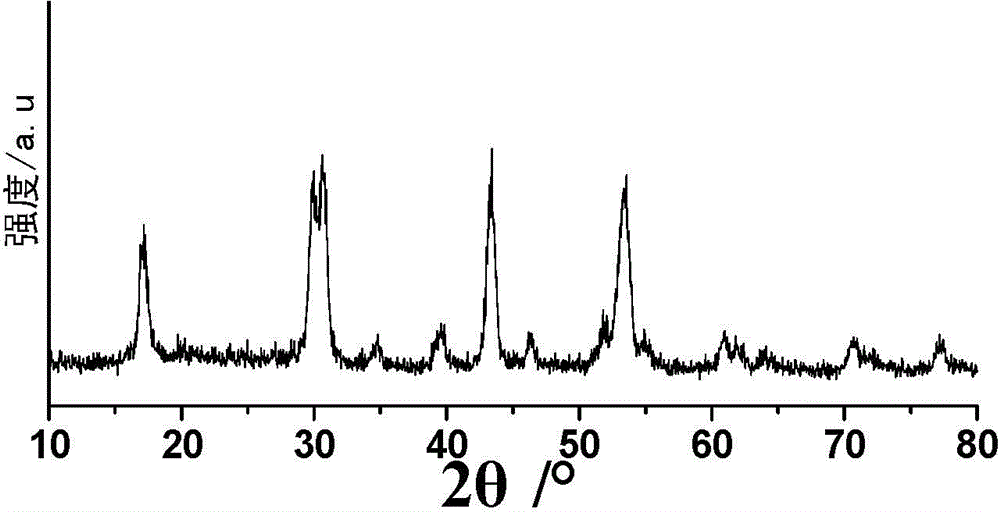
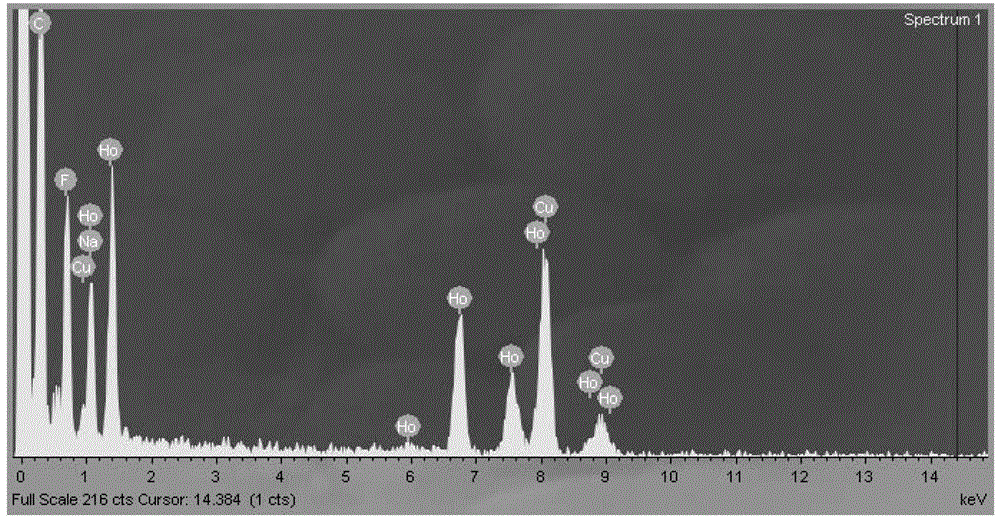
[0063] Weigh 2mmol (758.74mg) HoCl respectively 3 ·6H 2 O, dissolved in 2 mL of deionized water for later use; respectively add 15 mL of oleic acid and 30 mL of octadecene into a three-neck flask, then add the pre-prepared aqueous solution of chloride containing rare earth ions, and stir at room temperature for 2 hours; Argon to remove the air in the reaction flask; under the protection of argon atmosphere, heat slowly (the temperature rise rate is controlled at 30°C / hour), raise the temperature to 160°C, and keep it warm for 1 hour to remove the water in the system; stop heating, and naturally cool down to Room temperature; then dropwise add 200mg NaOH and 296.3mg NH 4 10 mL of methanol solution of F, stirred at room temperature for 3 hours to obtain a yellow-white solution; continue to pass in argon, and stirred at 120°C for 2 hours to remove methanol in the reaction system; after removing methanol, connect the condenser tube and raise the temperature To about 275°C, keep ...
Embodiment 2
[0073] Medical Imaging Application Effect Experiment
[0074] 1. MR imaging
[0075] 1.1 Experimental materials and instruments:
[0076] The UFCAs hydrophilic particle that embodiment 1 makes;
[0077] MR imaging detection instrument model: GE Signa 1.5T, GE Signa 3.0T and Agilent, 7T / 160
[0078] 1.2 Experimental animals: Kunming rats, with an average weight of 20g, purchased from the animal room of Fudan University School of Medicine;
[0079] 1.3 Experimental method: the influence of the UFCAs aqueous solution prepared in Example 1 with different concentrations on the transverse relaxation time of hydrogen protons at 1.5T / 3.0T / 7.0T; after the mice were intraperitoneally anesthetized with chloral hydrate, the thigh Contrast agent (2.5Ho mg / mL, ~30μL) imaging contrast effect at 3.0T / 7.0T; tail vein injection of contrast agent (dose 12mg Ho / kg), observe the MR contrast effect of the liver;
[0080] 1.4 Experimental results:
[0081] Figure 8 UFCAs hydrophilic particle ...
Embodiment 3
[0086] Toxicity evaluation experiment
[0087] 1. In vitro cytotoxicity test
[0088] 1.1 Experimental materials:
[0089] The UFCAs hydrophilic nano-particles that embodiment 1 makes;
[0090] 1.2 Experimental method:
[0091] MTT (3-(4,5-dimethylthiazol-2-y1)-2,5-diphenyltetrazolium bromide) method was used to evaluate the cell survival rate, and the specific experimental methods were: (1) inoculating cells: obtained with 10% fetal calf serum The culture medium was made into a single cell suspension, and 10 5 -10 6 Cells were inoculated into a 96-well plate, with a volume of 100 microliters per well. (2) Cell culture: After adding nanoparticles and co-cultivating the cells for 1 day, add MTT solution (5 mg / ml, prepared with PBS, pH=7.4) 50 to each well. Microliter, continue to co-cultivate for 4 hours, carefully aspirate and discard the culture supernatant in the well, for suspension cells, centrifuge and then aspirate and discard the culture supernatant in the well. (...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com