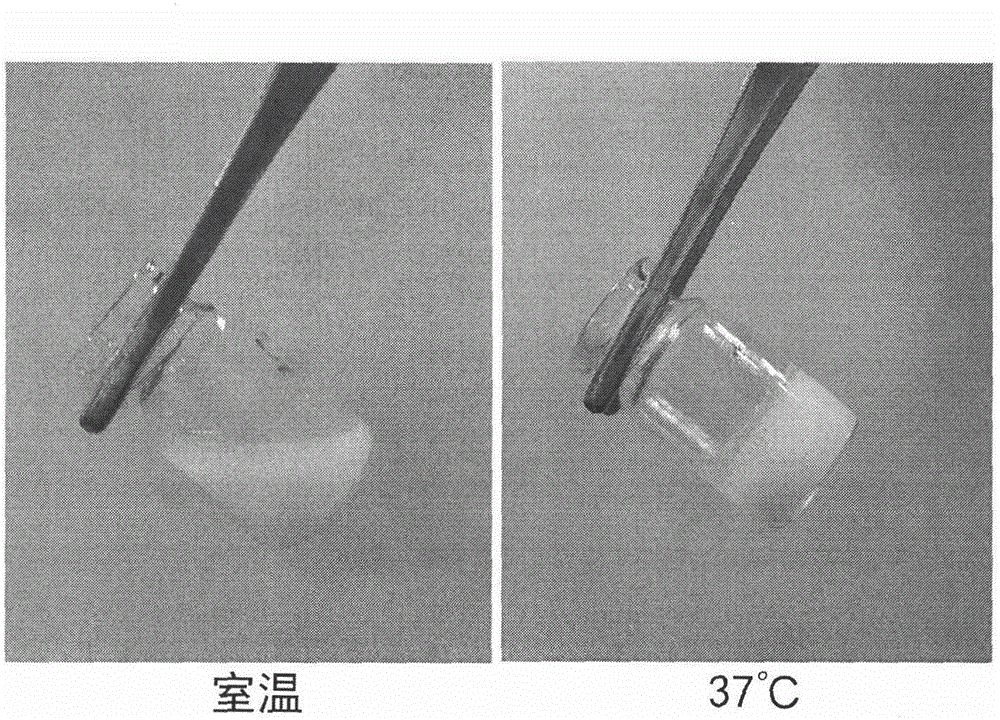
Temperature-sensitive in-situ gel preparation composition for anticular injection and preparation method thereof
An in-situ gel and composition technology, applied in the field of pharmaceutical preparations, can solve the problems of diclofenac sodium with short half-life, large side effects, gastrointestinal tract and kidney side effects, etc., to facilitate industrial production, improve compliance, and improve drug efficacy Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment 1
[0028] Weigh 0.2 g of diclofenac sodium and dissolve it in 10 mg / mL poloxamer 407 solution, add 0.2 g of sodium alginate and stir for 1 hour until completely dissolved, then add 30 mL of liquid paraffin containing 2.5% emulsifier, and stir with a high-speed disperser at 4000 rpm 3min, make emulsion A. Add 5mL of 25% calcium chloride solution to 15mL of liquid paraffin containing 2.5% emulsifier, and stir with a high-speed disperser at 4000rpm for 3min to prepare emulsion B. Add emulsion B dropwise to emulsion A, stir magnetically for 30 minutes to complete the cross-linking reaction, separate the microspheres by suction filtration, and freeze-dry to obtain drug-loaded sodium alginate microsphere freeze-dried powder. The particle size of the drug-loaded sodium alginate microsphere is 1-20 μm, and the drug-loaded amount is about 25%.
[0029] Chitosan (0.02g) was dissolved in 1mL glacial acetic acid solution (0.1M), and under the condition of stirring in an ice-water bath, β-so...
specific Embodiment 2
[0030]Weigh 0.2 g of diclofenac sodium and dissolve it in 10 mg / mL poloxamer 407 solution, add 0.2 g of sodium alginate and stir for 1 hour until completely dissolved, then add 30 mL of liquid paraffin containing 2.5% emulsifier, and stir with a high-speed disperser at 4000 rpm 3min, make emulsion A. Add 5mL of 25% calcium chloride solution to 15mL of liquid paraffin containing 2.5% emulsifier, and stir with a high-speed disperser at 4000rpm for 3min to prepare emulsion B. Add emulsion B dropwise to emulsion A, stir magnetically for 30 minutes to complete the cross-linking reaction, separate the microspheres by suction filtration, and freeze-dry to obtain drug-loaded sodium alginate microsphere freeze-dried powder. The particle size of the drug-loaded sodium alginate microsphere is 1-20 μm, and the drug-loaded amount is about 25%.
[0031] Chitosan (0.02g) was dissolved in 1mL glacial acetic acid solution (0.1M), and under the condition of stirring in an ice-water bath, β-sod...
specific Embodiment 3
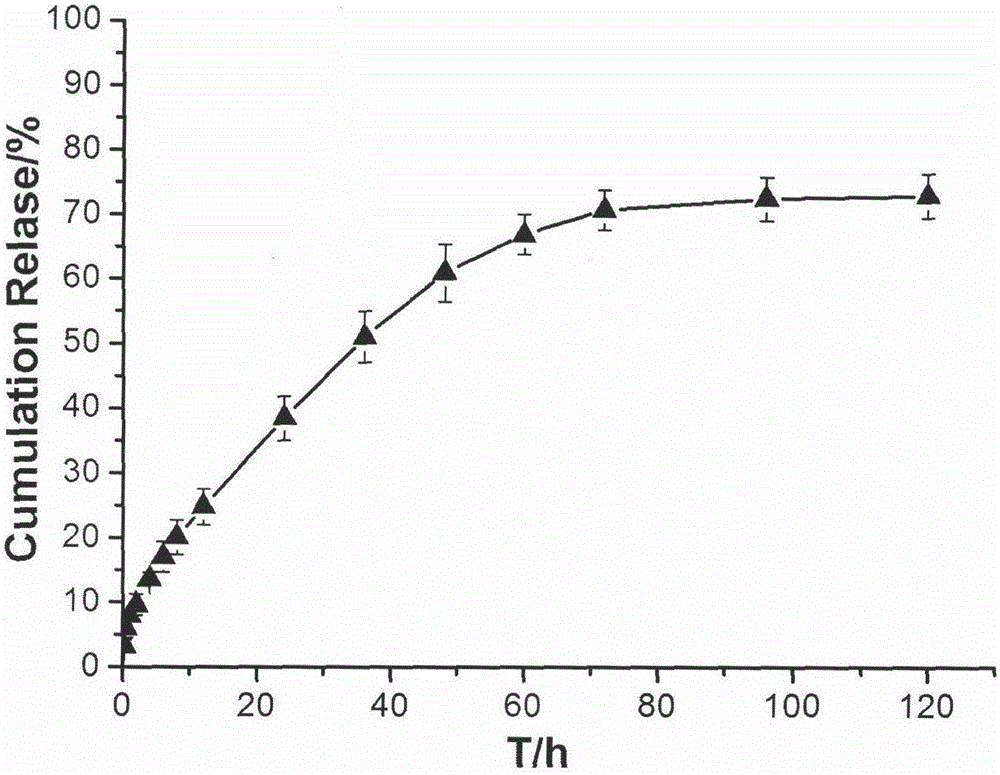
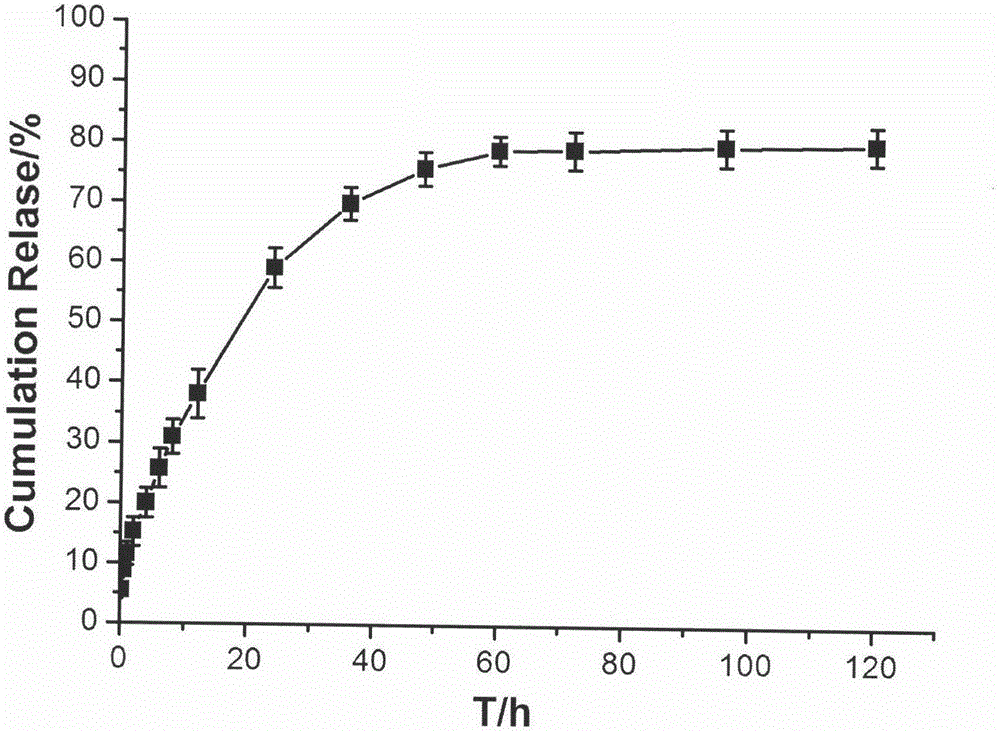
[0032] The temperature-sensitive in situ gel formulation composition for joint cavity injection obtained in Specific Example 1 of the present invention was subjected to an in vitro drug release test, and the results of its in vitro drug release curve are shown in the attached figure 2 shown.
[0033] Test method: Accurately measure 1mL of the composition obtained in Example 1, add it to a 10mL stoppered test tube, and place it in a water bath at 37±1°C for 1 hour. 37±1°C, shaking in a 100rpm shaker. Take out all the release medium at fixed sampling time points (0.25, 0.5, 1, 2, 4, 6, 8, 12, 24, 36, 48, 60, 72, 96, 120h), and add the same volume of fresh release medium . Filter through a 0.45 μm filter membrane, take the filtrate, measure the absorbance at a wavelength of 275 nm according to ultraviolet spectrophotometry, and calculate the cumulative drug release rate.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com