Industrial production method of esomeprazole
A technology of esomeprazole sodium and a production method is applied in the field of industrialized production of esomeprazole sodium, can solve problems such as large product loss, difficulty in stratification, inability to carry out industrialized production, etc., and achieves reduction in production energy consumption and The effect of raw material cost, saving production cost and environmental cost, high economic value and social benefit
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] 1. the preparation of formula II:
[0026]
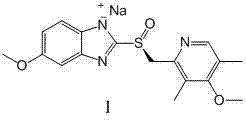
[0027] Add 125Kg of dichloromethane, 11.0Kg (62.5mol) of 2-mercapto-5-methoxybenzimidazole, 13.8Kg of 2-chloromethyl-3,5-dimethyl-4-methoxypyridine hydrochloride (62.1mol), tetrabutylammonium bromide 400g, lower the temperature to 0-20°C, add dropwise sodium hydroxide solution (33.2kg) with a mass concentration of 20%, drop it within 30min, and heat up to 20-30°C Insulation reaction for 3-5 hours, HPLC confirms that the content of 2-chloromethyl-3,5-dimethyl-4-methoxypyridine hydrochloride of the raw material is less than 1.0%, let it stand for stratification, remove the lower organic layer, and saturated saline Wash, concentrate the solvent to a quarter of its volume, cool down in an ice-salt bath (step by step, takes 1hr) to 0°C, keep stirring for 2hrs, centrifuge to get a wet product, wash with 2.0Kg n-heptane, 50±5°C Blast drying (-0.09MPa) yielded 15.4±0.5Kg of white solid, omeprazole sulfide represented by formula I...
Embodiment 2
[0034] 1. the preparation of formula II:
[0035]
[0036] Add 125Kg of dichloromethane, 11.0Kg (62.5mol) of 2-mercapto-5-methoxybenzimidazole, 13.8Kg of 2-chloromethyl-3,5-dimethyl-4-methoxypyridine hydrochloride (62.1mol), benzyltriethylammonium chloride 400g, lower the temperature to 0-20°C, add dropwise sodium hydroxide solution (33.2kg) with a mass concentration of 20%, and finish dropping within 30min, then heat up to 20-30 Insulate and react at ℃ for 3 to 5 hours, HPLC confirms that the raw material is less than 1.0%, let stand and separate, remove the lower organic layer, wash with saturated saline, concentrate the solvent to a quarter of its volume, and cool down in a circulating ice-salt bath (cooling in stages, consumption 1hr) to 0°C, keep stirring for 2hrs, centrifuge to get wet product, sprinkle wash with 2.0Kg n-heptane, blow dry at 50±5°C (-0.09MPa), get 15.0±0.5Kg white solid, the yield is 83.0% . The melting point of the product is 118-120°C, the purity ...
Embodiment 3
[0041] 1. the preparation of formula II:
[0042]
[0043] Add 125Kg of dichloromethane, 11.0Kg (62.5mol) of 2-mercapto-5-methoxybenzimidazole, 13.8Kg of 2-chloromethyl-3,5-dimethyl-4-methoxypyridine hydrochloride (62.1mol), 400g of tetrabutylammonium chloride, lower the temperature to 0-20°C, add dropwise sodium hydroxide solution (33.2kg) with a mass concentration of 20%, and finish dropping within 30min, then heat up to 20-30°C Insulation reaction for 3-5 hours, HPLC confirms that the raw material is less than 1.0%, let stand and separate layers, remove the lower organic layer, wash with saturated saline, concentrate the solvent to a quarter of its volume, and cool down in an ice-salt bath (step by step cooling, time-consuming 1hr ) to 0°C, keep stirring for 2 hours, centrifuge to get wet product, sprinkle wash with 2.0Kg n-heptane, and blow dry at 50±5°C (-0.09MPa) to obtain 15.2±0.5Kg white solid with a yield of 84.0%. The melting point of the product is 118-120°C, th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com