Praseodymium and holmium co-doping lead fluoride, alkali and yttrium up-conversion luminescent material as well as preparation method and application thereof
A lead fluoride base, luminescent material technology, used in luminescent materials, chemical instruments and methods, semiconductor/solid-state device manufacturing, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0027] The preparation method of the above-mentioned praseodymium-holmium co-doped lead fluoride alkali yttrium up-conversion luminescent material comprises the following steps:
[0028] Step S11, according to aPbF 4 -bRF-cYF 3 :xPr 3+ ,yHo 3+ The stoichiometric ratio of each element is weighed as PbO 2 , R 2 CO 3 , Pr 2 o 3 , Pr 2 o 3 and Ho 2 o 3 Powder, wherein a is 0.1-0.24, b is 0.05-0.15, c is 0.55-0.7, x is 0.01-0.08, y is 0.01-0.06, R is lithium, sodium, potassium, rubidium and cesium at least one of the elements.
[0029] In this step, preferably, a is 0.2, b is 0.08, c is 0.65, x is 0.04, and y is 0.03.
[0030] Step S13, mixing and dissolving the powder weighed in step S11 evenly in an acidic solvent for crystallization treatment to obtain crystals, dissolving the crystals in the solvent, and then adding ammonia water to adjust the pH value to 1-6 to obtain a mixed solution.
[0031] In this step, preferably, the acidic solvent includes hydrofluoric ac...
Embodiment 1
[0042] Choose PbO 2 , Li 2 CO 3 , Y 2 o 3 , Pr 2 o 3 and Ho 2 o 3 The powder is mixed according to the number of moles of each component being 0.2mmol, 0.08mmol, 0.65mmol, 0.02mmol and 0.015mmol. After mixing, dissolve in a hydrofluoric acid solvent for crystallization treatment to obtain crystals, then dissolve the crystals in distilled water and add ammonia water to the solution to adjust the pH value to 5. Then the mixed solution was transferred to a stainless steel reaction kettle lined with polytetrafluoroethylene, and kept at 300°C for 3 hours to obtain a precipitate. Then the obtained precipitate was repeatedly washed with ethanol and distilled water, evaporated to dryness at 100°C, put into a muffle furnace and burned at 950°C for 3 hours to obtain the general chemical formula 0.2PbF 4 -0.08LiF-0.65YF 3 : 0.04Pr 3+ , 0.03Ho 3+ up-converting phosphors.
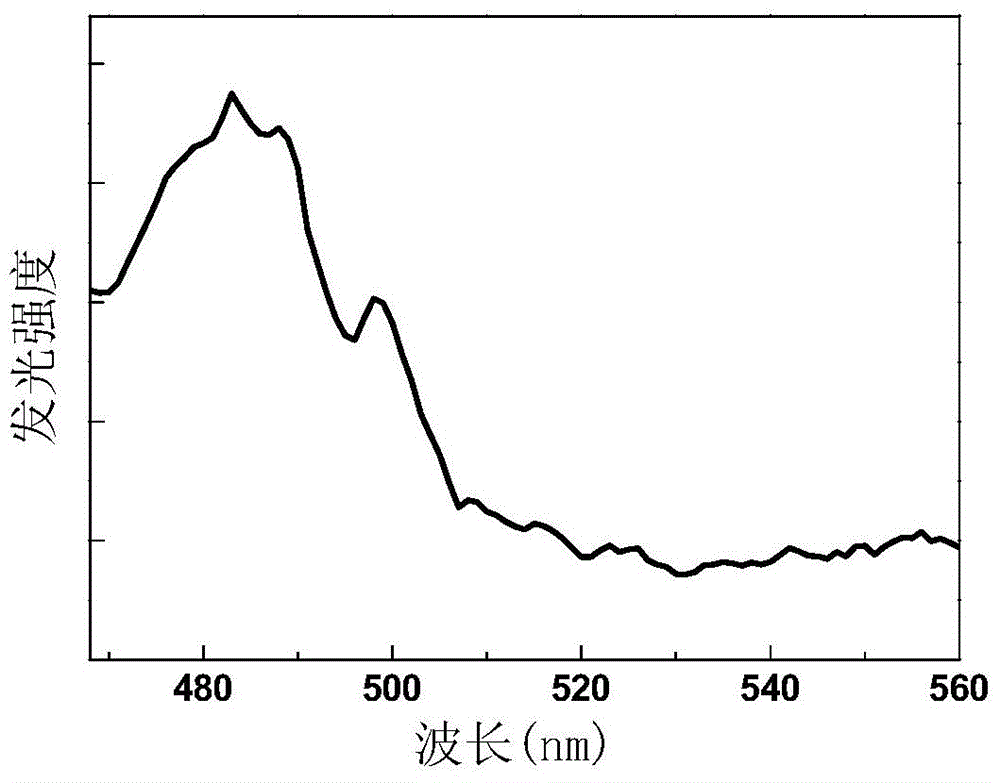
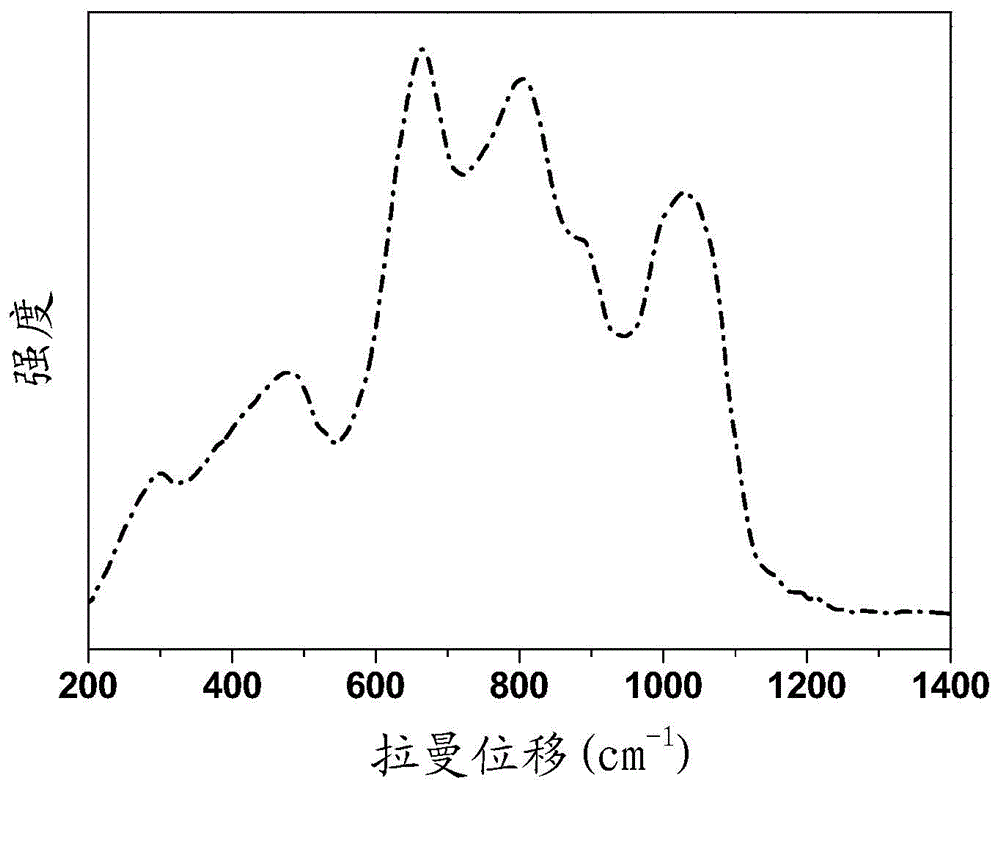
[0043] see figure 2 , figure 2 Shown is the general chemical formula of the praseodymium-holmium co-...
Embodiment 2
[0048] Choose PbO 2 , Li 2 CO 3 , Y 2 o 3 , Pr 2 o 3 and Ho 2 o 3 The powder is mixed according to the number of moles of each component being 0.1mmol, 0.15mmol, 0.73mmol, 0.005mmol and 0.005mmol. After mixing, dissolve in a hydrofluoric acid solvent for crystallization treatment to obtain crystals, then dissolve the crystals in distilled water and add ammonia water to the solution to adjust the pH value to 5. Then the mixed solution was transferred to a stainless steel reaction kettle lined with polytetrafluoroethylene, and kept at 300°C for 3 hours to obtain a precipitate. Then the obtained precipitate was repeatedly washed with ethanol and distilled water, evaporated to dryness at 100°C, put into a muffle furnace and burned at 800°C for 2 hours to obtain the general chemical formula 0.1PbF 4 -0.15LiF-0.73YF 3 : 0.01Pr 3+ , 0.01Ho 3+ up-converting phosphors.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com