H102 peptide nasal liposome-type spray
A nasal spray and spray technology, which is applied in the direction of non-active ingredients of polymer compounds, nervous system diseases, pharmaceutical formulations, etc., can solve the problems of poor mucous membrane penetration and unstable H102 peptide, and achieve less irritation and increase in vivo Stability, favorable therapeutic effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Accurately weigh 30 g of hydrogenated soybean phosphatidylcholine (HSPC), 6 g of dipalmitoylphosphatidylcholine (DSPC), and 1 g of mPEG5000-DSPE in a 1000 mL round-bottomed flask, add 100 mL of chloroform to fully dissolve the phospholipid material, and then evaporate to A thin film of phospholipids formed on the bottom of the flask. In addition, accurately weigh 6g of H102 peptide, dissolve it in 50mL of phosphate buffer (pH 6.0) containing 0.2% human serum albumin, add it into the flask to fully dissolve the phospholipid film, continue to add phosphate buffer to 200mL, hydrate, shake , ultrasonication, and membrane passage to obtain the H102 peptide liposome suspension. Add 10% glucose as a freeze-dried scaffold, dissolve, filter, and subpackage into sterilized nasal spray bottles for freeze-drying; after freeze-drying, take out and add bottle caps with quantitative spray pumps, and store in airtight low temperature. Before use, add sterilized three-distilled water t...
Embodiment 2
[0037] Accurately weigh 25g of egg yolk lecithin, 6.25g of cholesterol, and 2.5g of mPEG2000-DSPE in a 1000mL round bottom flask, add chloroform to dissolve, and rotary evaporate to form a film. In addition, 2 g of H102 peptide was precisely weighed, dissolved in 50 mL of phosphate buffer solution (pH 6.5) containing 0.5% human serum albumin, added to the flask to fully dissolve the phospholipid film, continued to add 100 mL of phosphate buffer solution, hydrated, shaken, Ultrasound and membrane passage to obtain H102 peptide liposome suspension. Add 5% trehalose as a freeze-dried scaffold agent, dissolve, filter through a 0.22 μm microporous membrane, pack into sterilized nasal spray bottles and freeze-dry to obtain H102 peptide nasal cavity liposome freeze-dried powder, which is sealed and stored at low temperature . Before use, add sterilized three-distilled water to dissolve, and then administer by spraying.
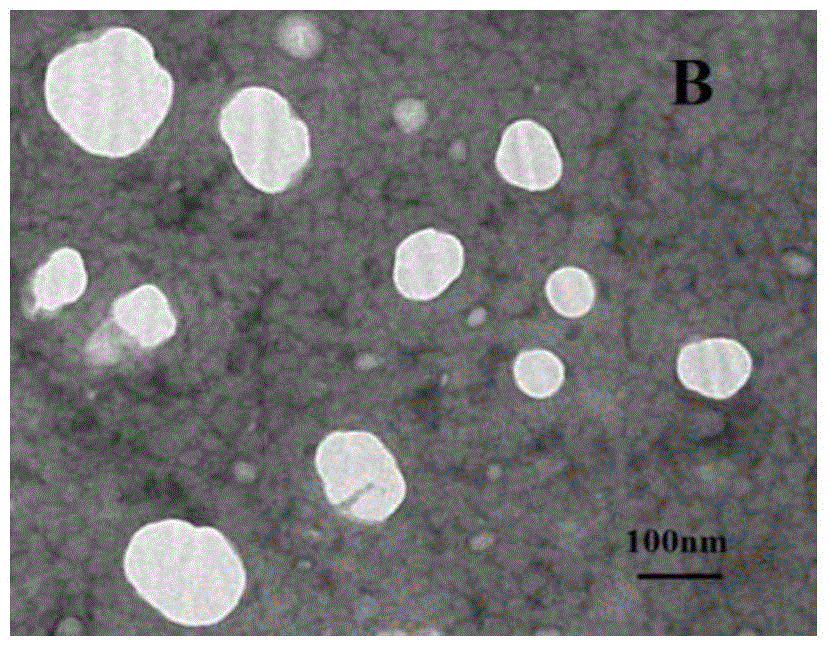
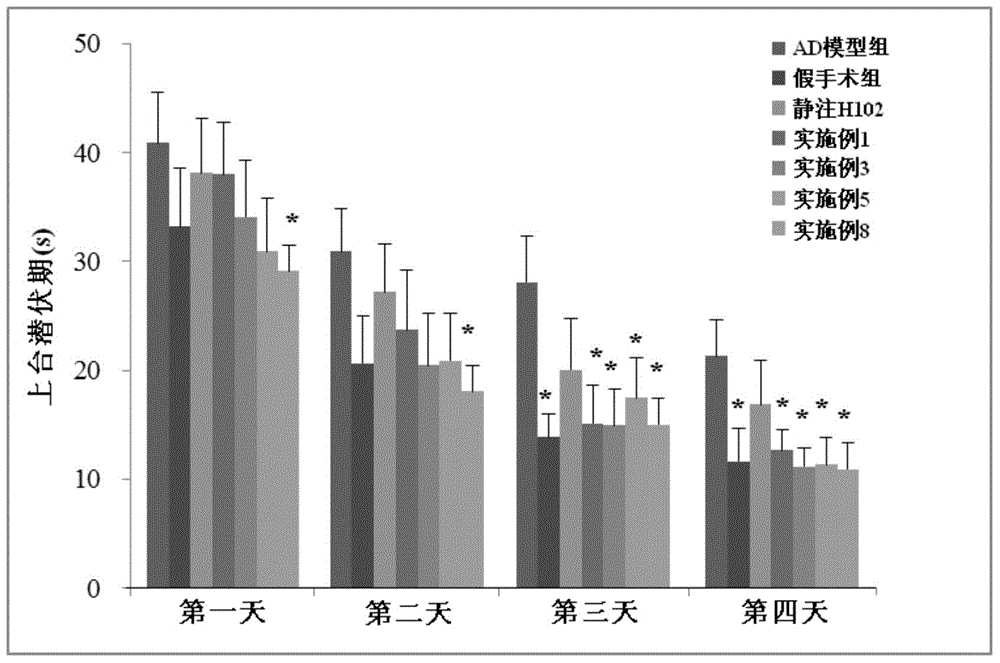
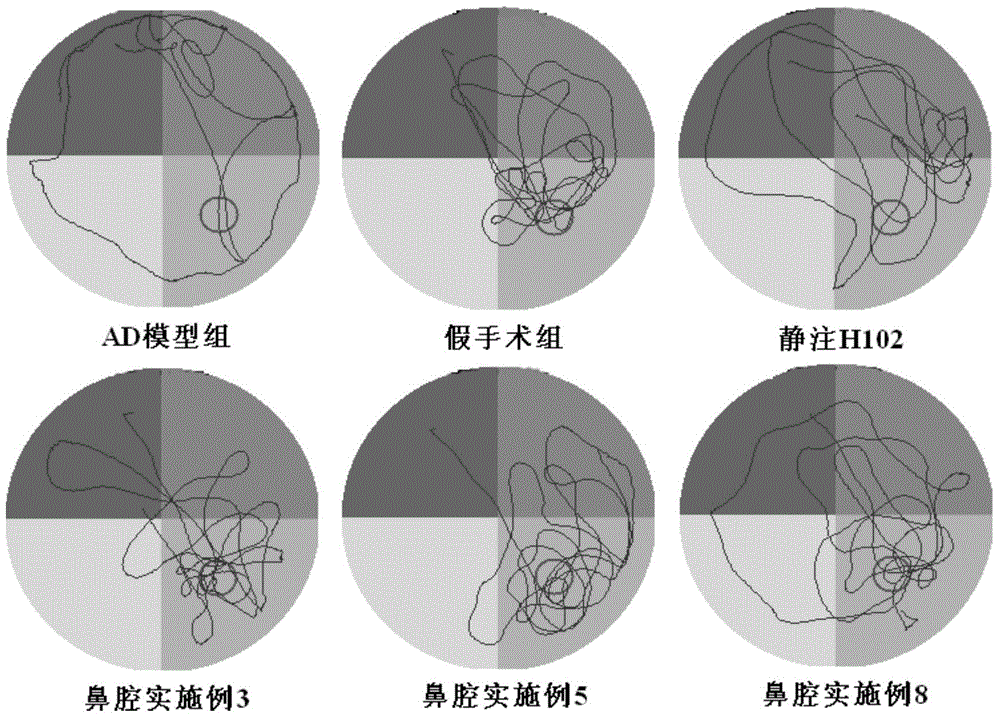
[0038] The H102 peptide nasal cavity liposome prepared in thi...
Embodiment 3
[0041]Accurately weigh 40 g of egg yolk lecithin, 10 g of cholesterol, and 2 g of mPEG2000-DSPE in a round bottom flask, add chloroform to dissolve, and rotary evaporate to form a film. In addition, 1 g of H102 peptide was accurately weighed, dissolved in 100 mL of phosphate buffer (pH 6.5) containing 0.1% human serum albumin, added to the flask to fully dissolve the phospholipid film, continued to add an appropriate amount of phosphate buffer, hydrated, oscillated, and ultrasonicated. 1. After being treated by a high-pressure homogenizer, pass through a membrane to obtain the H102 peptide liposome suspension. Add 10% trehalose to dissolve, filter, freeze-dry, take out, cover, and store in airtight low temperature. Before use, add sterilized three-distilled water to dissolve, and then administer by spraying.
[0042] The H102 peptide nasal cavity liposomes prepared in this example had an average particle size of 147.9 nm and an encapsulation efficiency of 71%; after freeze-dr...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com