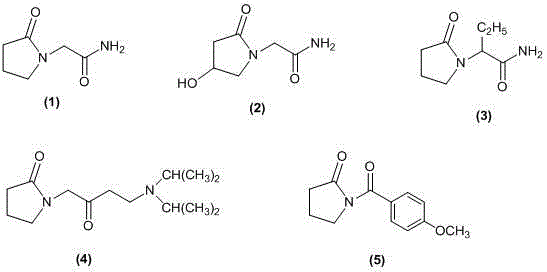
Preparation method for nebracetam
A technology of neraracetam and benzyl, which is applied in the field of preparation of neraracetam, can solve the problems of cumbersome operation, cumbersome post-processing, high risk of azidation reaction, etc., and achieve simple preparation process, good therapeutic effect, strong pharmacological effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
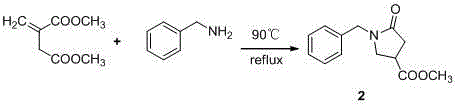
[0031] Methyl 1-benzyl-5-oxopyrrolidine-3-carboxylate 2 Synthesis
[0032]
[0033] Add 3.1g (50mmol) of dimethyl itaconic acid and 2.1g (50mmol) of benzylamine to a 50ml multi-necked bottle, under magnetic stirring, the oil bath is heated to 90°C, refluxed for about 21h, after the reaction is completed, a pale yellow liquid is obtained, After cooling to room temperature, it turned into a light yellow solid, 30 ml of dichloromethane and 30 ml of dilute hydrochloric acid were added to the reaction solution, the layers were shaken, and the organic phase was saturated with NaHCO. 3 The aqueous solution and the saturated NaCl aqueous solution were washed once each, and anhydrous NaSO 4 Dry, filter, evaporate dichloromethane, recrystallize from petroleum ether-ethyl acetate to obtain methyl 1-benzyl-5-oxopyrrolidine-3-carboxylate as a white solid 2 , the yield is 89%.
Embodiment 2
[0035] 1-Benzyl-4-hydroxymethyl-pyrrolidin-2-one 3 Synthesis
[0036]
[0037] Weigh 0.10 g (0.43 mmol) of methyl 1-benzyl-5-oxopyrrolidine-3-carboxylate 2 Put it in a 25ml multi-necked bottle, add 5ml methanol to dissolve, when the reaction solution is cooled to below 0°C, slowly add NaBH under stirring 4 (0.05g, 3eq) solid, after adding, continue the reaction at 0-5°C, the solution is colorless and has a small amount of white insoluble matter, TLC monitors the reaction process, and stops the reaction after the spot plate confirms that the reaction raw material disappears. , after the reaction is complete, slowly add 1M HCl dropwise to the reaction solution to pH=5-6, evaporate methanol under reduced pressure, add water to the residue, then extract three times with dichloromethane, wash once with water, combine the organic phases, no Dry over sodium sulfate, filter, spin-dry the filtrate to obtain a viscous liquid, and cool to obtain a yellow solid 1-benzyl-4-hydroxymeth...
Embodiment 3
[0039] In a 50ml three-necked flask equipped with a thermometer, add 0.95g (4.08mmol) of methyl 1-benzyl-5-oxopyrrolidine-3-carboxylate 2 Dissolve in 12ml methanol to get a colorless clear liquid, add NaBH slowly at 10-15℃ 4 Solid (0.46g, 3eq), after the addition, the reaction was incubated at 10-15°C, and the reaction was monitored by TLC. After the reaction was completed, 1M HCl was slowly added dropwise to the reaction solution to pH=5-6, and evaporated under reduced pressure. Methanol, water was added to the residue, extracted three times with dichloromethane, washed once with water, combined the organic phases, dried over anhydrous sodium sulfate, filtered, and the filtrate was spin-dried to obtain a viscous liquid, which was cooled to obtain a yellow solid 1-benzyl-4-hydroxyl Methyl-pyrrolidin-2-one 3 0.81g, yield 96.9%, purity 97%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com