A kind of detection method of Shufengjiedu capsule
A technology of Shufengjiedu capsules and detection methods, which is applied in the direction of measuring devices, instruments, scientific instruments, etc., can solve the problems that it is difficult to reflect the overall quality of medicines, and achieve the advantages of comprehensive monitoring, good reproducibility, and high precision Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
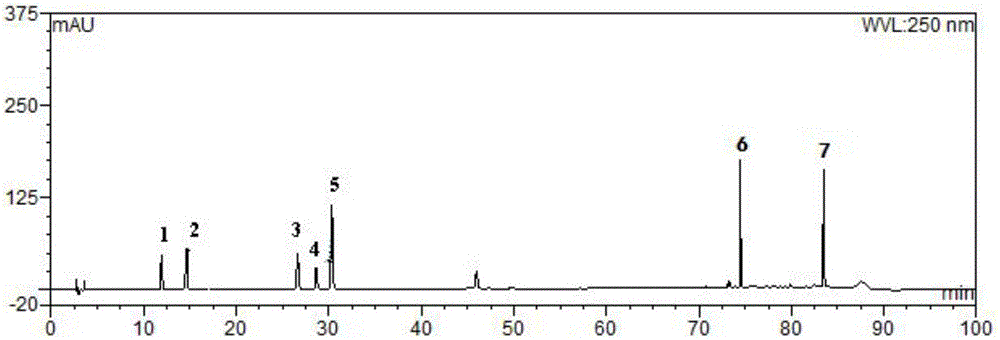
[0051] Embodiment 1 adopts HPLC to establish the multi-component assay method of Shufengjiedu capsule content
[0052] Preparation of mixed reference solution: Accurately weigh 4.80 mg of Verbenain, 4.46 mg of Verbenain, 5.96 mg of Polydatin, 2.94 mg of Forsythiaside A, 10.56 mg of Verbacoside, and 10.30 mg of Monoammonium Glycyrrhizinate , Emodin 2.66mg, respectively into 25ml measuring bottles, dissolved in methanol and diluted to the mark, shaken well, as a stock solution. Precisely measure 5ml each of the reference substance stock solution into a 10ml measuring bottle, dilute to the mark with methanol, and shake well to obtain a mixed reference substance solution.
[0053] Preparation of the test solution: Put the content of Shufeng Jiedu Capsules in a mortar and grind it into powder, weigh 1.000g of the powder, put it in a 100ml round bottom flask, add 25ml of 70% ethanol aqueous solution, weigh, reflux and extract for 2h, after cooling Weigh, make up the weight loss w...
Embodiment 2
[0061] The investigation of need testing solution preparation method in the multi-component detection method of embodiment 2
[0062] 1. Inspection of extraction methods
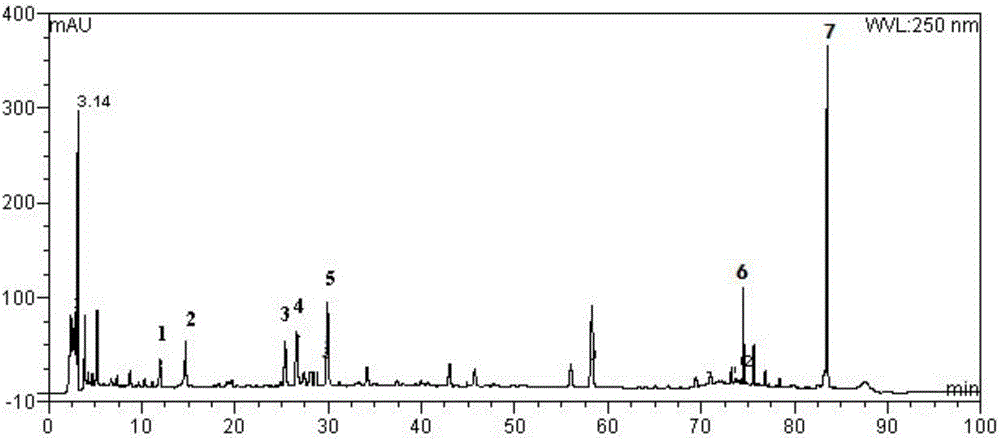
[0063] Weigh 3 parts of the contents of Shufengjiedu capsules, add appropriate amount of 70% ethanol, respectively adopt ultrasonic, reflux, Soxhlet extraction, and filter to obtain the test solution, and carry out the test under the determined conditions. The content calculation results of the relevant components are shown in table 3.
[0064] Table 3 Extraction method inspection results
[0065]
[0066] Comparing the three extraction methods, it can be seen that the reflux extraction has the highest efficiency, so the reflux extraction method is selected.
[0067] 2. Investigation of extraction solvent
[0068] Weigh 3 parts of 1.000g Shufengjiedu Capsule powder in parallel, add 25ml of 50%, 70%, and 90% ethanol solution respectively, reflux extraction for 2 hours, filter to obtain the test soluti...
Embodiment 3
[0084] Methodological investigation of embodiment 3 multi-component detection method
[0085] (1) Precision test
[0086] Adopt the operation and condition identical with embodiment 1, get about 1.000g of sample, accurately weigh, process by need testing solution preparation method, precision draw need testing solution 10 μ l, inject high performance liquid chromatograph, in above-mentioned chromatographic condition 6 consecutive injections under the following conditions, the RSD values of verbenin, verbenin, polydatin, forsythiaside A, verbascoside, monoammonium glycyrrhizinate, and emodin were 2.53%, 2.18%, 2.76%, and 2.87, respectively. %, 2.19%, 1.33%, 1.97%. The precision is good.
[0087] (2) Stability investigation
[0088] Using the same operation and conditions as in Example 1, take about 1.000 g of the sample, prepare and extract according to the test solution, inject samples at 0, 1, 3, 6, 9, 12, and 24 hours respectively, and measure under the above-mentione...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com