Attenuated human rotavirus strain with broad-spectrum immunogenicity and vaccine prepared from the attenuated strain
A human-derived rotavirus, immunogenic technology, applied in the direction of antiviral agents, inactivation/attenuation, virus antigen components, etc., can solve the safety problems of live attenuated vaccines, etc. Stable, high viral titer effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
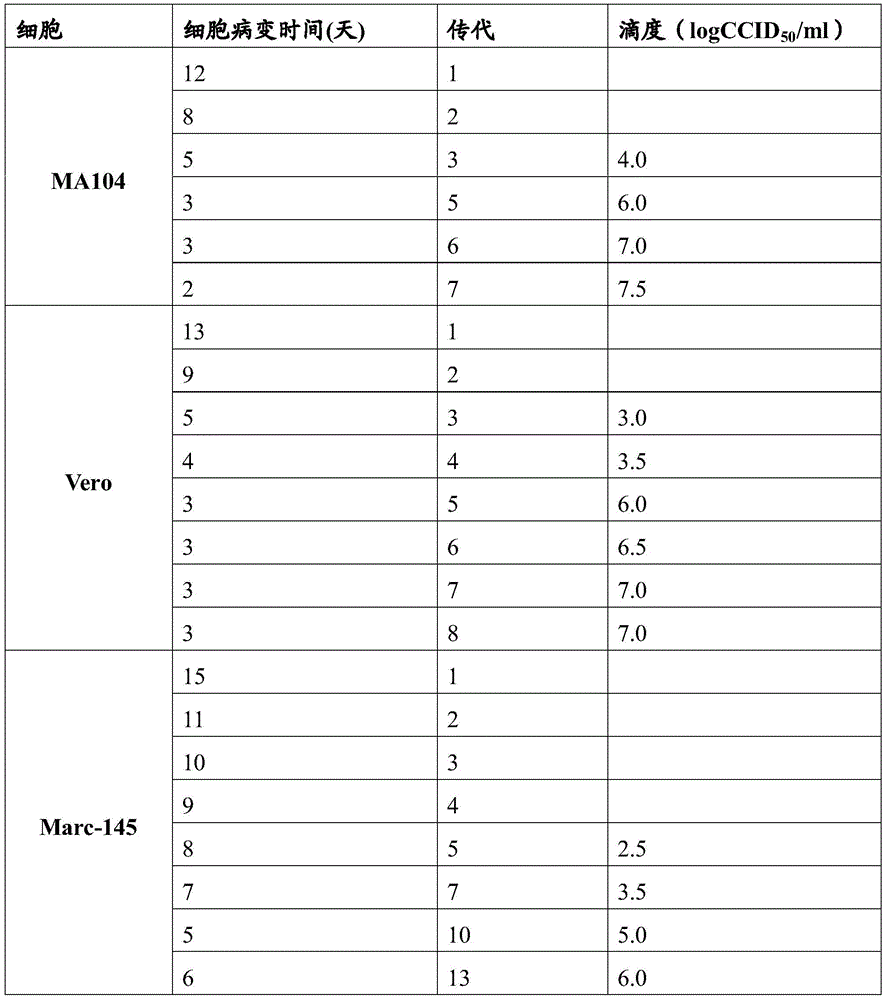
[0039]Example 1. Isolation, passage and screening of human rotavirus
[0040] In 2011, 10 feces samples of children with acute rotavirus diarrhea were collected in a hospital in China, resuspended with 20% PBS (pH=7.3), sterile filtered, centrifuged at 3000g for 20 minutes, and 0.1ml supernatant was added to trypsin (20ug / ml) mixed for 30 minutes, inoculated with PBS washed 3 times monolayer MA-104 cells (T-25 cell culture medium containing MEM, double antibody (100U gentamicin and 100ug streptomycin) and 8-10% fetal bovine serum ), incubate at 37°C for 1 hour, add virus maintenance solution (containing MEN and 0.5ug / ml trypsin) and incubate at 37°C for 10-15 days, freeze and thaw 3 times and centrifuge at 2000g for 20 minutes, take the supernatant and trypsin (20ug / ml / ml) mixed with MEM for 30 minutes and diluted 20 times with MEM to inoculate MA104 cells at 37°C for 8-9 days, and repeated passage for 8-10 generations until the virus incubation period was 2-3 days. The iso...
Embodiment 2
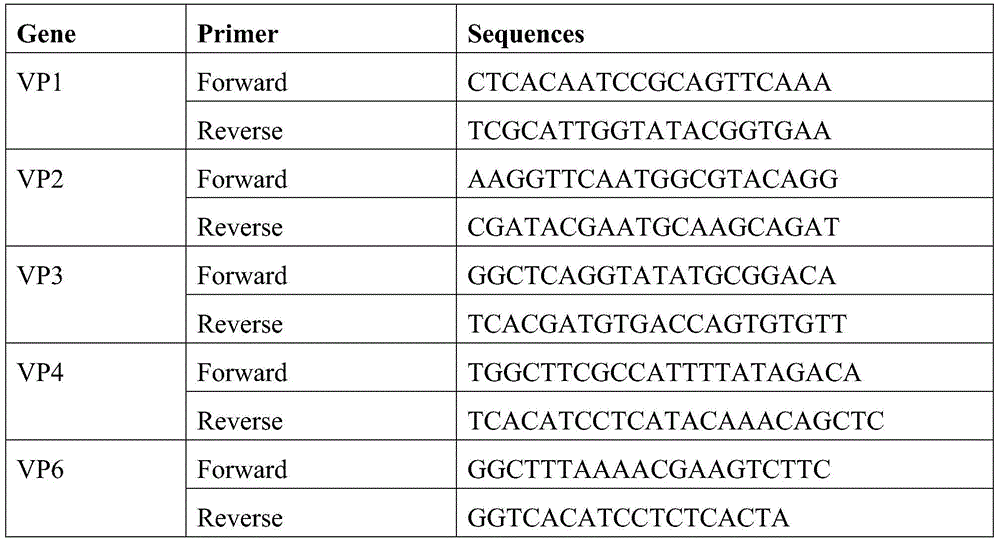
[0045] Identification and typing of embodiment 2 virus
[0046] The enriched virus HRV-LZ-2013 was typed by RT-PCR, and then confirmed by plaque reduction neutralization test. 45-50CCID 50 The virus was activated with 1.5ug of trypsin at 37°C for 1 hour, mixed with different dilutions of G1-G10, P2-P8 monoclonal antibodies or hyper-free serum to inoculate a 6-well plate of MA-104 cells grown into a monolayer, covering the 6-well plate containing 5ug / ml trypsin, 37°C, 5% CO 2 Incubate in the incubator for 4 days, fix with formaldehyde, count staining, and form 60% plaques, indicating the highest dilution of antibody.
[0047] The rotavirus was centrifuged at 36000rpm×3 hours, then the supernatant was removed, and the purified rotavirus HRV-LZ-2013 was obtained by centrifugation at 38000rpm×3 hours, 30% sucrose density, and Freund’s complete adjuvant and incomplete adjuvant were added to Rabbits were primed and boosted to prepare HRV-LZ-2013 hyper-immune serum.
[0048] Pur...
Embodiment 3
[0055] The preparation of embodiment 3 human rotavirus attenuated live vaccines
[0056] Include the following steps:
[0057] (1) Proliferation and culture of virus
[0058] Vero cells were inoculated at 200,000-300,000 cells / ml in spinner bottles, and after 3 days of culture at 37°C, rotavirus HRV-LZ-2013 or its 20-100 generation Vero cell subcultures were inoculated at MOI 0.01-0.1, and cultured at 37°C for 1 Hours later, wash with normal saline and add virus maintenance solution, the virus maintenance solution is MEM containing 0.1% bovine serum, pH 7.2, cultured at 32°C for 3-5 days, repeated freezing and thawing 3 times, centrifuging to remove cell debris, using MA104 cells were used to determine the virus titer, and the titer was ≥5.5logCCID 50 / ml and sterile as a qualified vaccine stock solution;
[0059] (2) Purification of rotavirus HRV-LZ-2013
[0060] Using continuous sucrose density gradient centrifugation, centrifuge at 120,000g in TNE buffer containing 60% ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com