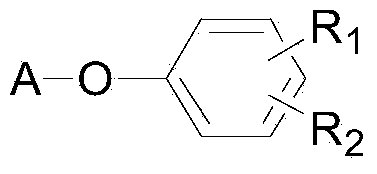
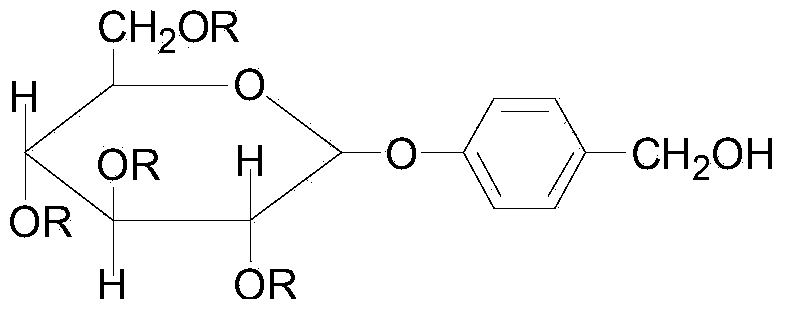
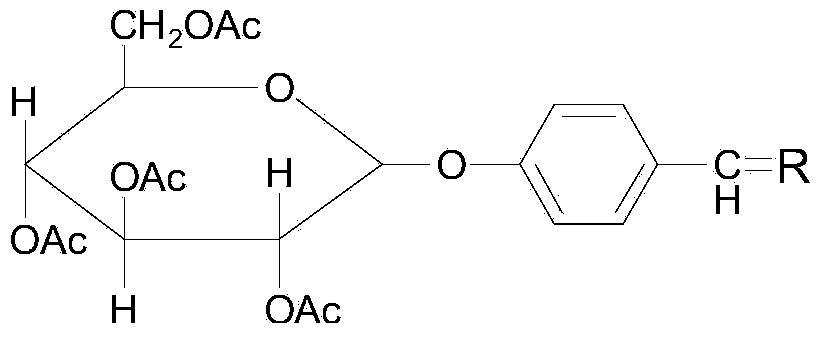
Gastrodin ferulate compound and preparation method and application of gastrodin ferulate compound
A technology of ester compound and ferulic acid ester, which is applied in the field of medicine and chemical industry
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0087] A gastrodin ferulic acid ester compound, the preparation of the compound shown in the formula (II) is realized by the following steps:
[0088] (1) At 32°C, add anhydrous glucose to the catalyst perchloric acid and acetylate it with acetic anhydride to generate full acetyl sugar. TLC detects the end point of the reaction. After the acetylation is completed, hydrogen bromide (gas) is introduced, and the temperature is controlled at At 28°C, hemiacetal hydroxy bromide of peracetyl sugar is used to synthesize bromotetraacetylglucose, and the molar ratio of feed is anhydrous glucose: acetic anhydride: perchloric acid: hydrogen bromide = 1: 7.5: 0.04: 0.76;
[0089] (2) Add phase transfer catalyst tetrabutylammonium bromide, carbonate and p-hydroxybenzaldehyde in the two-phase system composed of chloroform and water with a volume ratio of 1:1, control the temperature at 55°C, bromotetraacetylglucose Dissolve in chloroform and add dropwise. The weight ratio of bromotetraacety...
Embodiment 2
[0095] In the specific implementation of the present invention, a gastrodin ferulic acid ester compound, said gastrodin ferulic acid ester compound is a compound shown in formula (I), and its preparation method is:
[0096] Preparation of compounds of formula (Ic)
[0097] Acetylgastrodin 4.6g (i.e. 10.13mmol), pyridine 1.2g (i.e. 15.20mmol), DMAP0.4g (i.e. 3.04mmol) and 100ml dichloromethane shown in formula (II) are dropped into the reactor, stirred, and another Acetyl feruloyl chloride 3.9g (i.e. 15.20mmol) shown in formula (III) was dissolved in 100ml methylene chloride, then the acetyl feruloyl chloride methylene chloride solution was dripped into the reactor, stirred at room temperature for 2 hours, and then the Wash with 3% hydrochloric acid and saturated ammonium chloride solution, dry over anhydrous magnesium sulfate, filter, evaporate the solvent under reduced pressure to obtain the compound (5.9 g, 93%) shown in formula (Ic), 1 H NMR (400MHz, CDCl 3 )δ7.66(d, J=16...
Embodiment 3
[0103] A kind of gastrodin ferulic acid ester compound of the present invention, described this compound is the compound shown in formula (1b), and its preparation method is:
[0104] Put 2.0g (2.97mmol) of the compound represented by formula (Ic) into 40ml of diethylamine, stir at 60°C, wait until the dissolution is complete, add 40ml of toluene to dilute, then wash with 1M sulfuric acid and saturated ammonium chloride, and anhydrous sulfuric acid Sodium drying, filtering, steaming toluene, chromatographic silica gel column ethyl acetate-petroleum ether volume ratio 1: 1 mixed together as mobile phase, isolated compound 428mg shown in formula (Ib), 1 H NMR (400MHz, DMSO) δ9.60(s, 1H), 7.57(d, J=15.9Hz, 1H), 7.41–7.28(m, 3H), 7.12(d, J=8.2Hz, 1H), 7.04 (d, J=8.5Hz, 2H), 6.79(d, J=8.2Hz, 1H), 6.51(d, J=15.9Hz, 1H), 5.31(d, J=4.7Hz, 1H), 5.14(s ,2H),5.08(d,J=4.4Hz,1H),5.02(d,J=5.2Hz,1H),4.88(d,J=7.2Hz,1H),4.55(t,J=5.7Hz,1H ),3.81(s,3H),3.78–3.62(m,1H),3.46(dt,J=11.8,6.0Hz,1H),...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com