Method for preparation of Li2FeSiO4 and Li2FeSiO4/C anode material
A lithium iron silicate and carbon positive electrode technology, which is applied in battery electrodes, electrical components, electrochemical generators, etc., can solve problems such as hindering industrial production, product agglomeration, and local heating too fast, so as to reduce synthesis conditions and raw materials The effect of cost, high cycle specific capacity, and good cycle stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] (1) Add tetraethyl orthosilicate and ferric nitrate in a molar ratio of 1:1 to a mixed solution of a certain proportion of absolute ethanol and deionized water to form solution A, and the concentration of orthosilicate is 0.16 molL -1 ; Add polyethylene glycol 200 and ammonia water to a mixed solution of a certain proportion of absolute ethanol and deionized water to form solution B, and the concentration of polyethylene glycol 200 is 0.08 molL -1 , the pH value of solution B is 10;
[0032] (2) During the rapid stirring of solution B, pour into solution A to form a precipitate;
[0033] (3) Add lithium acetate with a relative stoichiometric ratio to the mixed solution obtained in step (2), mix well and place it under 80 ° C water bath conditions to evaporate and dry, and finally obtain a reddish-brown precursor;
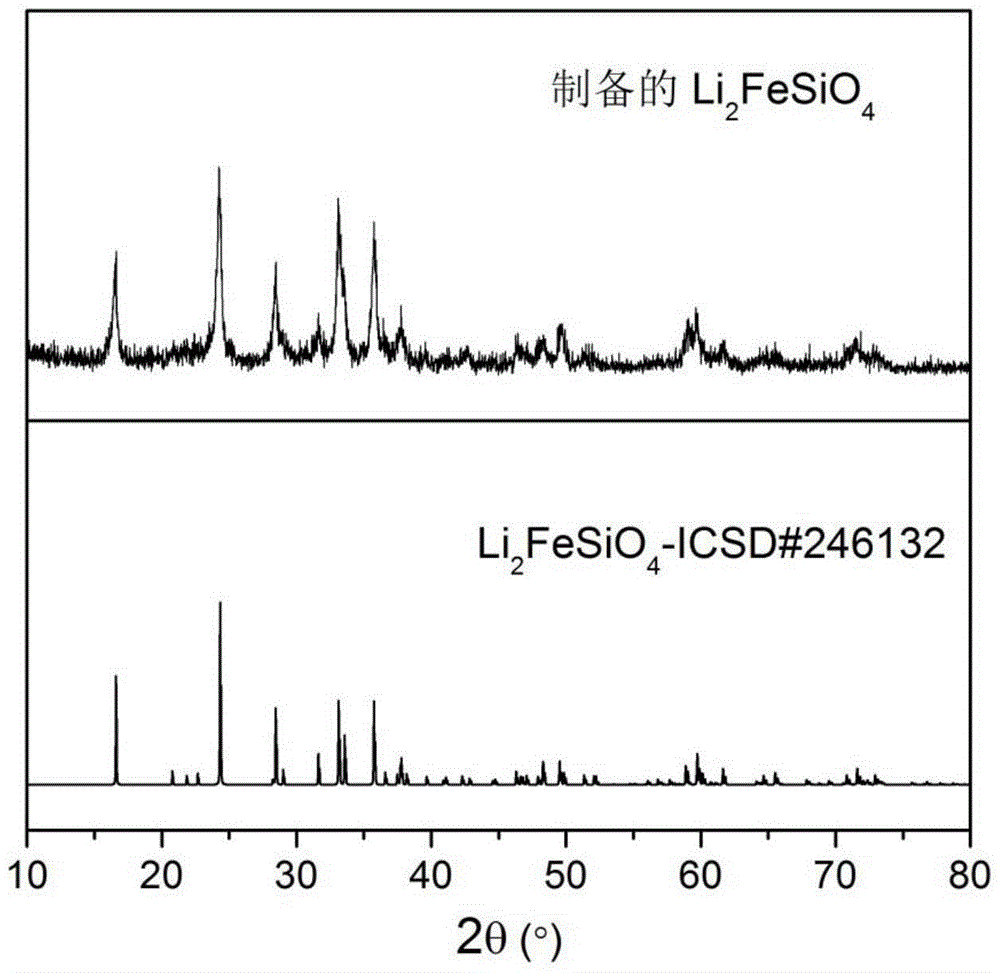
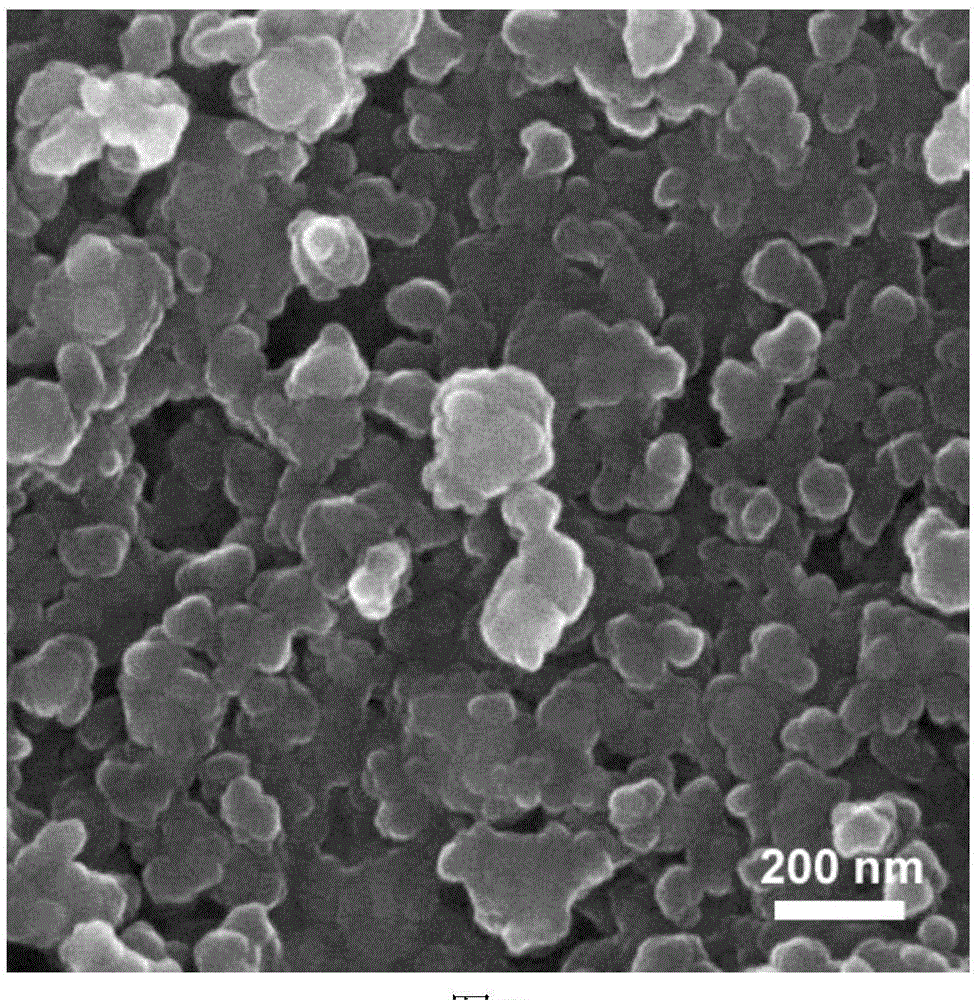
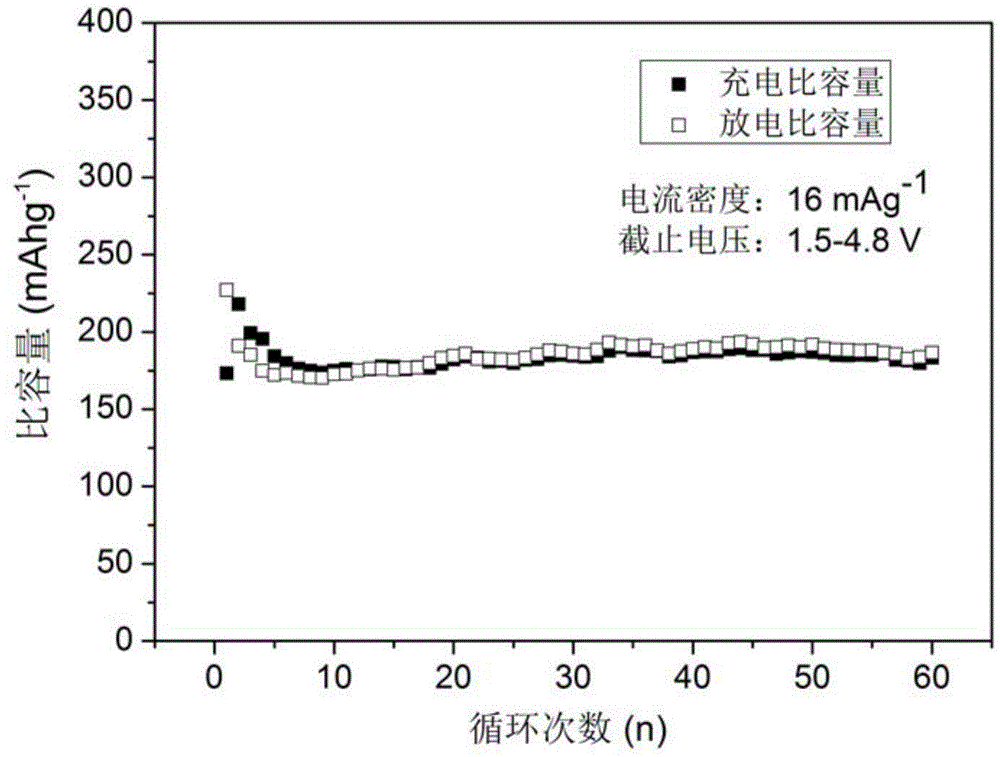
[0034](4) The precursor in step (3) was calcined at 700 °C for 10 h in a mixed gas of hydrogen and argon, and after cooling, lithium iron silicate / carbon (L...
Embodiment 2
[0037] (1) Add silicon tetrachloride and ferric nitrate to a mixed solution of a certain proportion of absolute ethanol and deionized water in a molar ratio of 1:1 to form a solution A with a silicon tetrachloride concentration of 0.16 molL -1 ; Add polyethylene glycol 200 and ammonia water to a mixed solution of a certain proportion of absolute ethanol and deionized water to form solution B, and the concentration of polyethylene glycol 200 is 0.08 molL -1 , the pH value of solution B is 10;
[0038] (2) During the rapid stirring of solution B, pour into solution A to form a precipitate;
[0039] (3) Add lithium acetate with a relative stoichiometric ratio to the mixed solution obtained in step (2), mix well and place it under 80 ° C water bath conditions to evaporate and dry, and finally obtain a reddish-brown precursor;
[0040] (4) The precursor in step (3) was calcined at 700 °C for 10 h in an argon atmosphere, and after cooling, lithium iron silicate / carbon (Li 2 FeSiO ...
Embodiment 3
[0043] (1) Add tetraethyl orthosilicate and iron oxalate in a molar ratio of 1:1 to a mixed solution of a certain proportion of absolute ethanol and deionized water to form solution A, and the concentration of orthosilicate is 1 molL -1 ; Add polyethylene glycol 200 and ammonia water to a mixed solution of a certain proportion of absolute ethanol and deionized water to form solution B, and the concentration of polyethylene glycol 200 is 0.2 molL -1 , the pH value of solution B is 10;
[0044] (2) During the rapid stirring of solution B, pour into solution A to form a precipitate;
[0045] (3) Add lithium acetate with a relative stoichiometric ratio to the mixed solution obtained in step (2), mix well and place it under 80 ° C water bath conditions to evaporate and dry, and finally obtain a reddish-brown precursor;
[0046] (4) The precursor in step (3) was calcined at 900 °C for 10 h in an argon atmosphere, and after cooling, lithium iron silicate / carbon (Li 2 FeSiO 4 / C) C...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com