Method used for separating and identifying flavonoid matters in tobacco by adopting liquid chromatography-mass spectrography
A liquid chromatography and identification method technology, applied in the field of plant extract separation and identification, can solve problems such as limited sensitivity of ultraviolet detectors, achieve simple and rapid sample preparation, improve detection efficiency, and good accuracy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] ——Isolation and Identification of Isorhamnetin in Tobacco
[0045] (1) Experimental reagents and devices
[0046] Acetonitrile (chromatographic grade), methanol (chromatographic grade), and ethanol (chromatographic grade) were purchased from Merck, Germany. Ultrapure water was produced by a Millipore purification system. Other standard samples were purchased from Sigma-Aldrich, Alfa Aesar and Bailingwei. Waters UPLC liquid chromatograph (USA), AB SCIEX 5500 triple quadrupole mass spectrometer (USA). SB-50D ultrasonic extractor (Ningbo Xinzhi Biotechnology Co., Ltd.), Waters BEH C18 (15 cm×2.1 mm×1.7 μm) reversed-phase chromatographic column (Waters, USA), MILLI-Q pure water machine (MILLIPORE) , LD5-2A centrifuge (Beijing Jingli Centrifuge Co., Ltd.), vortex mixer (Breda, Netherlands), CP2245 analytical balance (sensitivity 0.0001g, Sartorius, Germany).
[0047] (2) Experimental materials
[0048] The mature middle leaves and petals of Yunyan 97, Honghua Dajinyuan,...
Embodiment 2
[0056] ——Isolation and identification of isorhamnetin-3-O-rutinoside from tobacco
[0057] (1) Experimental reagents and devices
[0058] With embodiment 1.
[0059] (2) Experimental materials
[0060] With embodiment 1.
[0061] (3) Experimental method
[0062] With embodiment 1.
[0063] (4) Experimental results
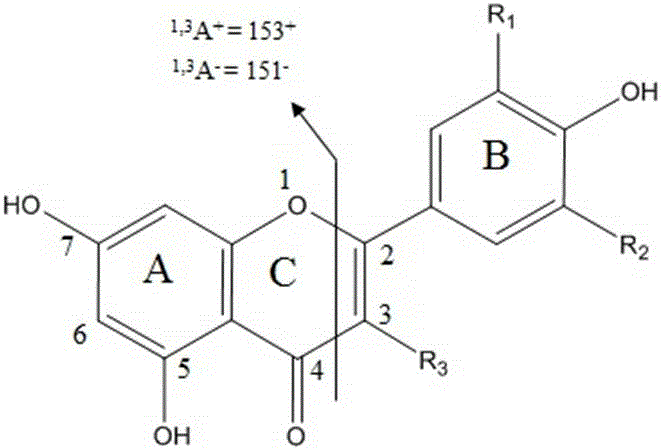
[0064] Since isorhamnetin was found in tobacco samples, it was hypothesized that glycosyl-bound compounds of isorhamnetin also exist in tobacco. The molecular ion of isorhamnetin (315 - ) was used as a characteristic ion to perform a full scan of the parent ion, and it was found that there was an obvious chromatographic peak at the retention time of 4.55 min ( Figure 4 ), the detected precursor ion is 623 - . According to 623 - with 315 - There is a neutral loss of 308, and the compound is preliminarily judged to be isorhamnetin-rutinoside. Collect 4.55 min at 623 - Ion secondary fragment ion, and submit it to the mass bank website (www.massbank.jp) f...
Embodiment 3
[0066] ——Isolation and Identification of Quercetin-3-O-Rutinoside from Tobacco
[0067] (1) Experimental reagents and devices
[0068] With embodiment 1.
[0069] (2) Experimental materials
[0070] With embodiment 1.
[0071] (3) Experimental method
[0072] With embodiment 1.
[0073] (4) Experimental results
[0074] at 151 - When scanning precursor ions for characteristic ions, there is an obvious chromatographic peak at the chromatographic retention time of 5.80 min, and the detected precursor ion is 301 - . Collect 301 at 5.80 min -Ion secondary fragment ion, and submit it to the mass bank website (www.massbank.jp) for spectral library comparison, and found that it is very similar to the secondary mass spectrum of quercetin in the spectral library, buy quercetin The standard substance of the compound was compared with the retention time and the secondary mass spectrum of the compound to be identified, and it was confirmed that it was quercetin. Molecular...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com