Method for preparation of GLP-1 polypeptide or analogue thereof through MFH fusion protein and application of GLP-1 polypeptide or analogue thereof
A GLP-1 and fusion protein technology, applied in the biological field, can solve the problems of low yield, harsh production conditions, and single source, and achieve the effect of simple purification steps, high recovery rate, and mild conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] Embodiment 1 fusion protein MFH-DP-H6-E7-GLP-1 and MFH-DP-H6-E7-R34 vector construction and expression
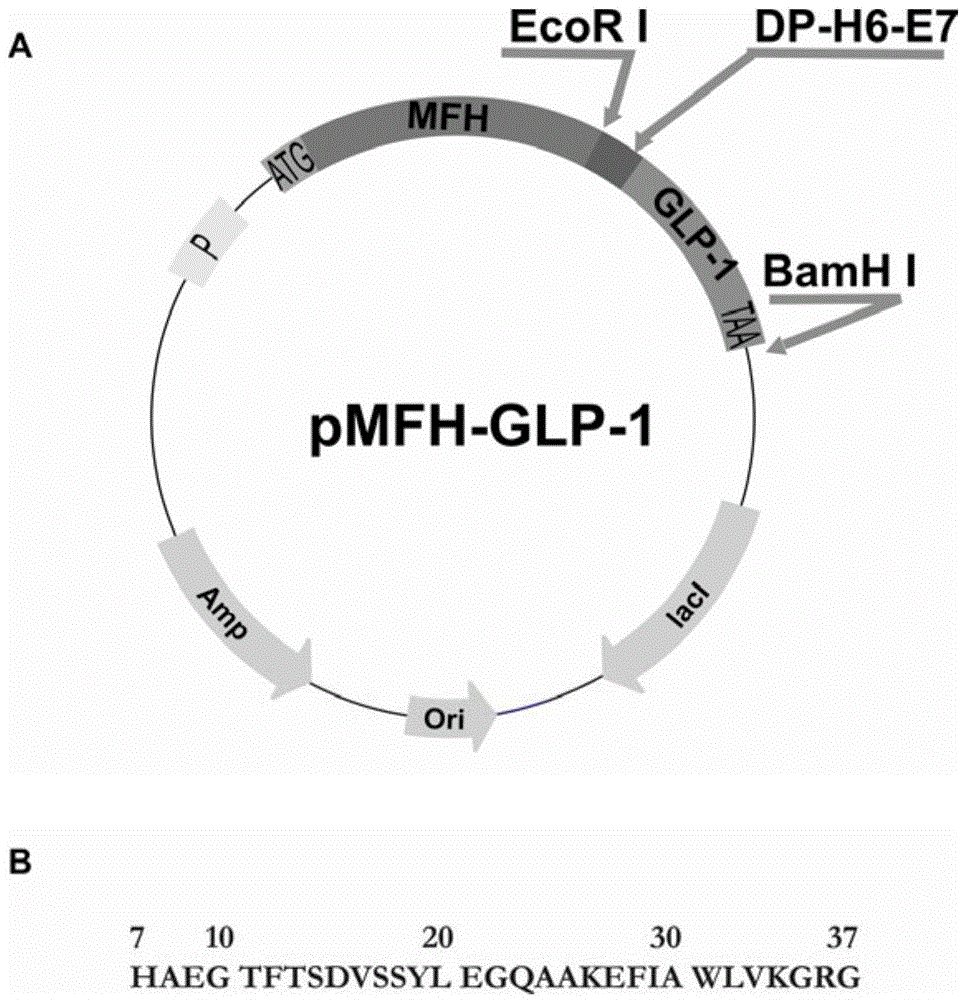
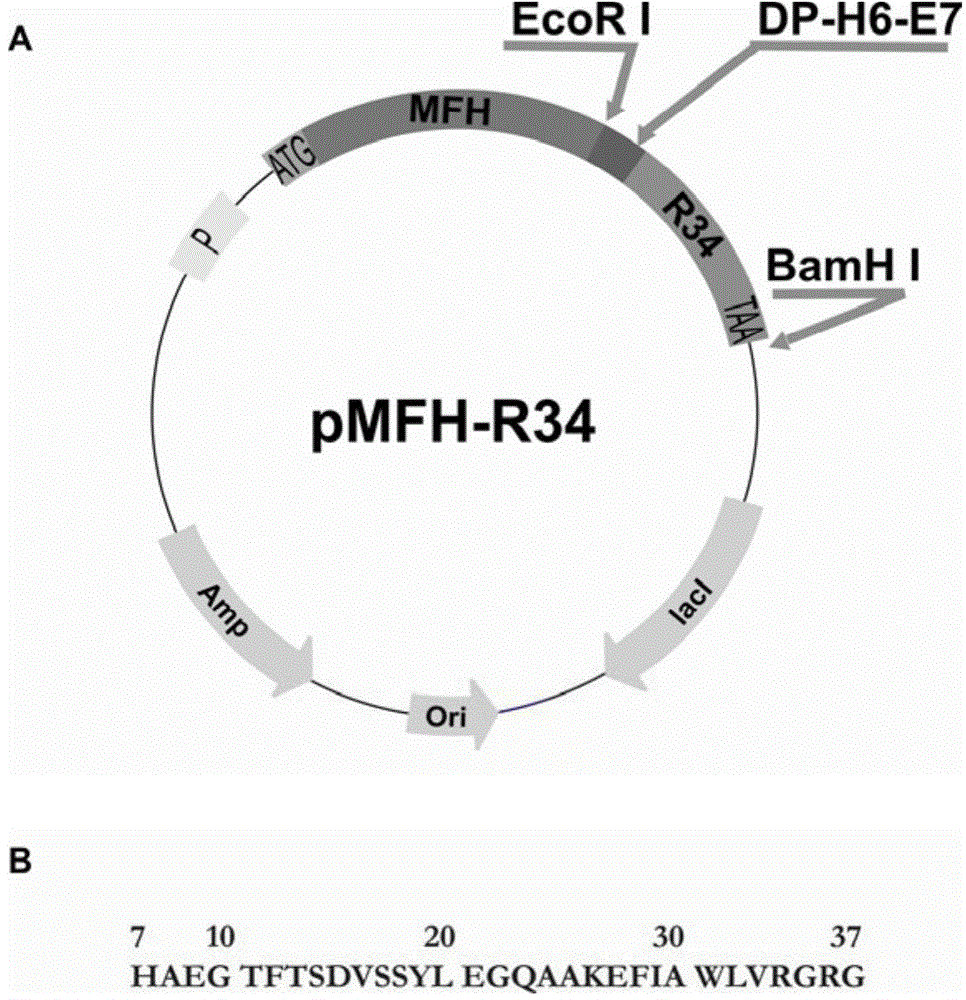
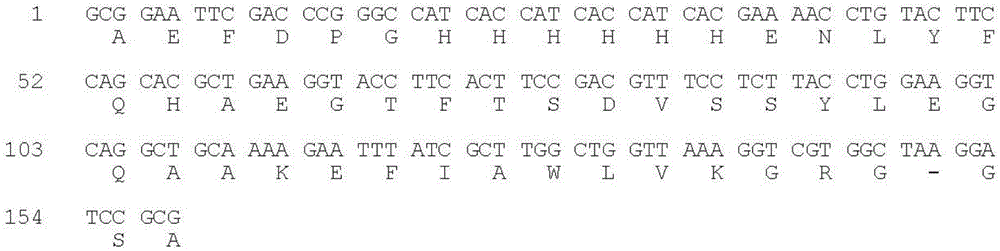
[0058] The codons preferred by Escherichia coli were used to design primers, and the overlap extension PCR method was used to plasmid pCMFH-GLP template (HJ Li, CX Zhou and JZ Su, Chemical ligation and cleavage on solid support facilitate recombinant peptide purification. Protein Expression and Purification50,238 –246,2006), amplified and constructed DNA sequences encoding DP-H6-E7-GLP-1 and DP-H6-E7-R34 fusion proteins respectively, and then, the DNA sequences of the two GLP-1 derivative peptides were passed After double digestion with EcoR I and BamH I, insert into the high-efficiency expression vector pMFH-MCS (HJ Li, CX Zhou and JZ Su, Protein Expression and Purification50,238–246,2006) that was cut with EcoR I and BamH I , constructed into prokaryotic expression recombinant plasmids of GLP-1 and its derivative peptides: pMFH-GLP-1 ( figure 1 ) and pMFH-R34 ( ...
Embodiment 2
[0064] Embodiment 2 Utilizes the fermentation method that lactose induces engineered bacteria to produce fusion protein
[0065] The present invention also determines the use of lactose as an inducer to induce the expression of the fusion protein. Because lactose itself cannot induce the initiation of the Lac promoter, it needs to be converted into allolactose through the action of β-galactosidase before it acts as an inducer. The invention enables engineering bacteria to express target fusion protein through seed cultivation and design of fermentation medium conditions. Specific steps are as follows:
[0066] (1) BL21 expressing fusion protein MFH-DP-H6-E7-GLP-1, MFH-DP-H6-E7-R34 was picked from the plate to 5 ml (containing ampicillin) LB medium, 200 rpm / min, 37°C, shake the flask for 12-16 hours. The composition of LB medium is as follows: yeast extract 5g / L, casein hydrolyzate 10g / L, sodium chloride 10g / L, initial pH 6.5-8.0.
[0067] (2) After cultivation, take 1 mil...
Embodiment 3
[0074] Example 3 Fusion protein ethanol precipitation purification
[0075] The present invention confirms that fusion proteins can be efficiently purified by ethanol precipitation. Collect the bacterial cells expressed by the lactose-induced fusion protein by centrifugation at 5000-6000 rpm for 15-30 minutes, resuspend the bacterial cells in PBS (containing 6M urea) at room temperature, and stir for 30 minutes. Minutes, then the broken cells were processed by a high-pressure homogenizer, the cell broken liquid was centrifuged at 12000 rpm for 20-30 minutes, and the total protein supernatant was obtained, and the supernatant was precipitated by ethanol for 2-8 hours (1:1 volume ratio; -20°C), high-speed centrifugation at 12,000 rpm to remove the precipitate (-20°C), and the secondary supernatant was subjected to ethanol precipitation for 8 to 12 hours (1:1 volume ratio; -20°C). 12000 revs / separation centrifuge collects precipitate, and secondary centrifugation precipitate is ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com