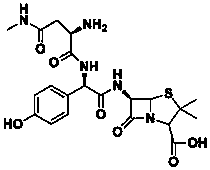
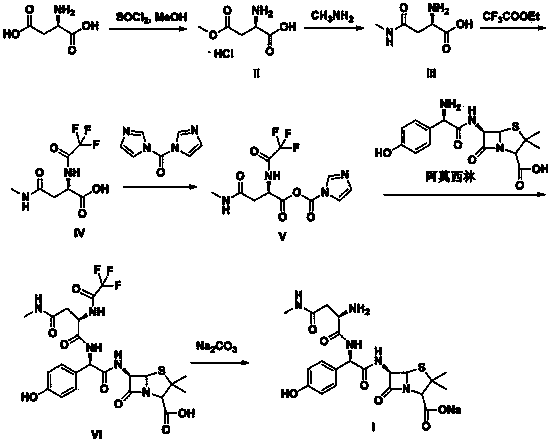
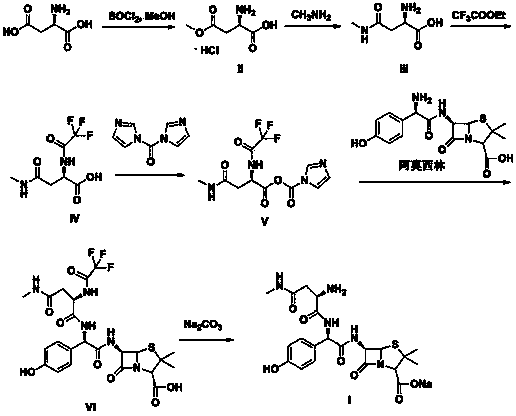
Convenient method for preparing aspoxicillin sodium
A technology for apocillin sodium and a compound, which is applied in the field of convenient preparation of apocillin sodium, can solve the problems of high toxicity of 2-nitrothiophenol, inability to guarantee production safety, strong odor, etc. The effect of low environmental pressure, low labor protection intensity and high productivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Synthesis of embodiment 1 formula II compound
[0033]Suspend D-aspartic acid (160g, 1.2mol) in 850mL of methanol, cool down to -5~0°C, slowly add thionyl chloride (178.4g, 1.5mol) dropwise, after the drop is complete, heat up to 25~35 ℃, stirred and reacted for 4-5 hours, after the reaction was completed, methanol was distilled off under reduced pressure, and the obtained white solid was added to 500 mL of ethyl acetate, stirred and washed for 0.5 hours, filtered, and dried to obtain 192 g of the compound of formula II, with a yield of 87%, mp: 185 ~186°C.
[0034]
Embodiment 2
[0035] The synthesis of embodiment 2 formula III compound
[0036] (2) Dissolve the compound of formula II (192g, 1.05mol) in 400mL of water, cool down to 0-5°C, add 350mL of 40% methylamine aqueous solution dropwise, after the drop is completed, heat up to 25-35°C, and stir for 12-14h , the reaction was completed, concentrated under reduced pressure, adjusted the pH to 5 with 1 mol / L hydrochloric acid, concentrated under reduced pressure to dryness, added 550 mL of acetone, stirred for 1 h, filtered a large amount of precipitated solids, and dried to obtain 127 g of the compound of formula III, with a yield of 83%. mp: 188-189°C.
[0037]
Embodiment 3
[0038] Synthesis of embodiment 3 formula IV compound
[0039] The compound of formula III (127g, 0.87mol) was dissolved in 500mL of methanol, triethylamine (96g, 0.95mol) was added, and ethyl trifluoroacetate (184.6g, 1.3mol) was added dropwise at a controlled temperature of 40-45°C. After completion, keep the temperature at 30-35°C for 14-16h. After the reaction is completed, concentrate under reduced pressure, add 500mL of dichloromethane, stir and crystallize at a temperature of 0-5°C for 1h, and collect the precipitated solid to obtain 164g of the compound of formula IV. 78%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com