Dopamine derivative, molecular imprinted polymer and preparation methods and application of dopamine derivative and molecular imprinted polymer
A technique of fluorescent molecular imprinting and dopamine, which is applied in the preparation of sulfonamides, chemical instruments and methods, and material excitation analysis, and can solve the problems of large sample loading, high detection limit, and low detection limit.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0053] The dopamine derivative provided by the invention, its preparation method comprises the following steps:
[0054] (a) Mix the fluorescent chromogenic agent precursor solution and the dopamine aqueous solution according to the molar ratio of the fluorescent chromogenic agent precursor to the dopamine precursor 1:2 to 2:1; the preferred fluorescent chromogenic agent precursor solution has a concentration of 5g / L to 10g / L dansyl chloride acetone solution, dansyl fluoride acetone solution; the preferred concentration of the dopamine aqueous solution is 20g / L to 40g / L.
[0055] (b) Add alkaline buffer solution to the mixed solution prepared in step (a), adjust the final pH value of the mixed solution between 9.0 and 10.0, and seal and avoid light at 50 to 80 degrees Celsius to make the mixed solution undergo sulfonation reaction to obtain the crude product of the dopamine derivative; the preferred alkaline buffer is a borax buffer with a pH value between 9 and 12.
[0056]...
Embodiment 1
[0070] A kind of dopamine derivative, is characterized in that, has the structure of formula (I):
[0071]
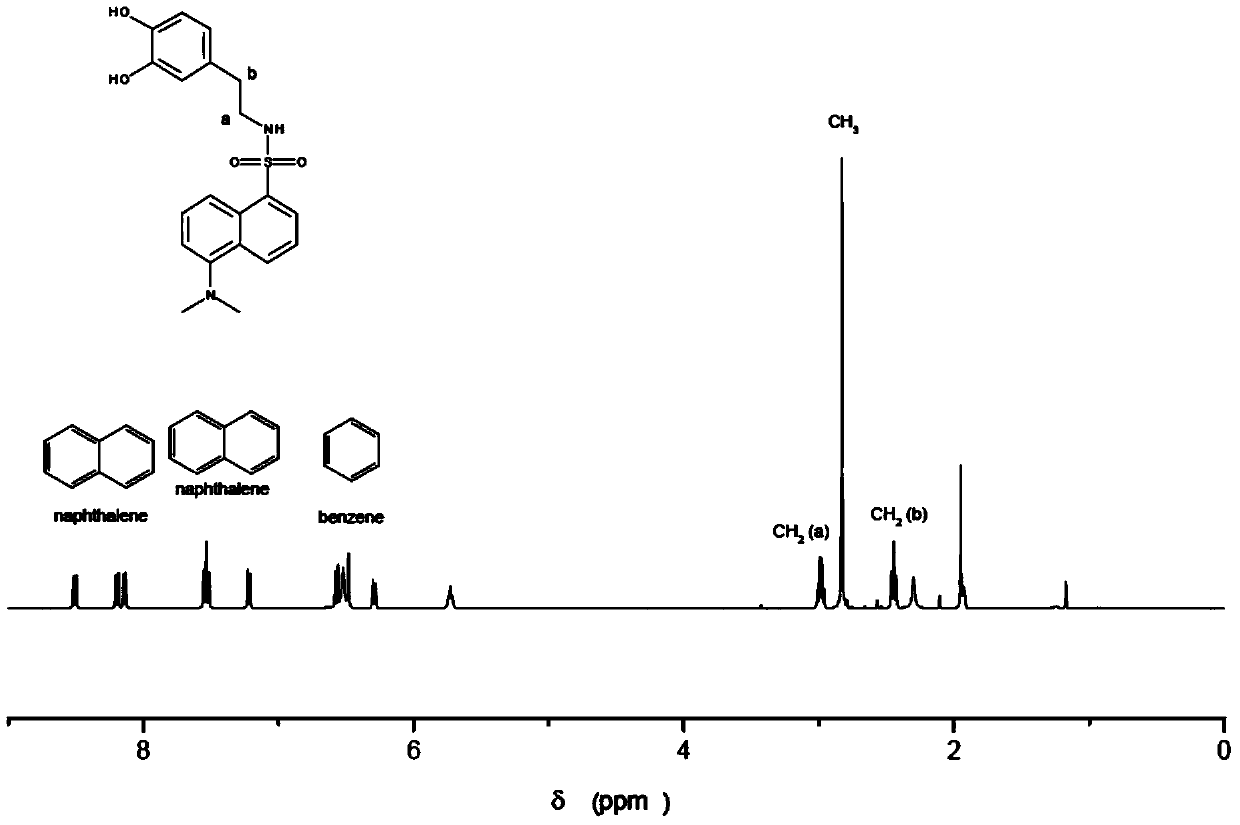
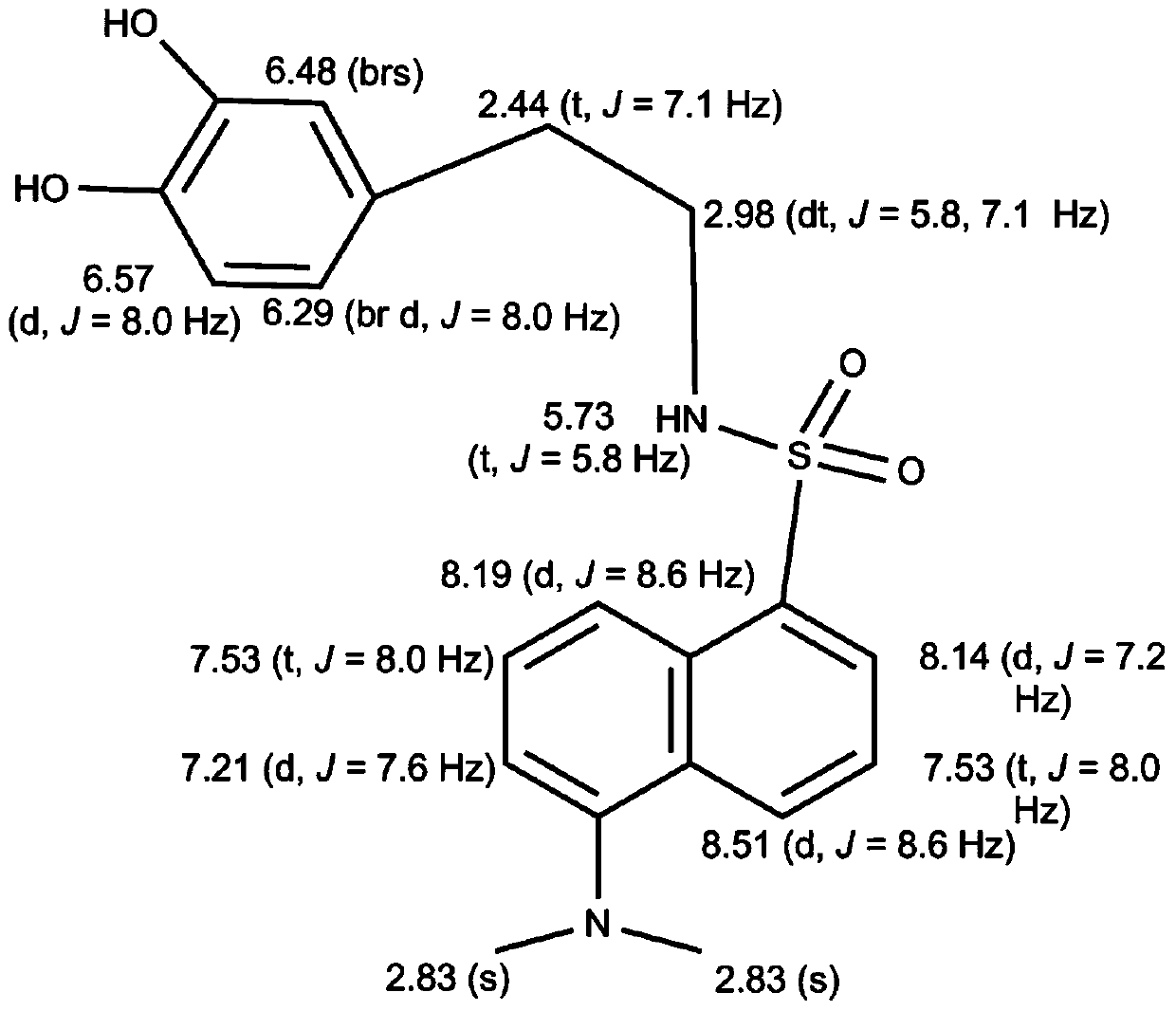
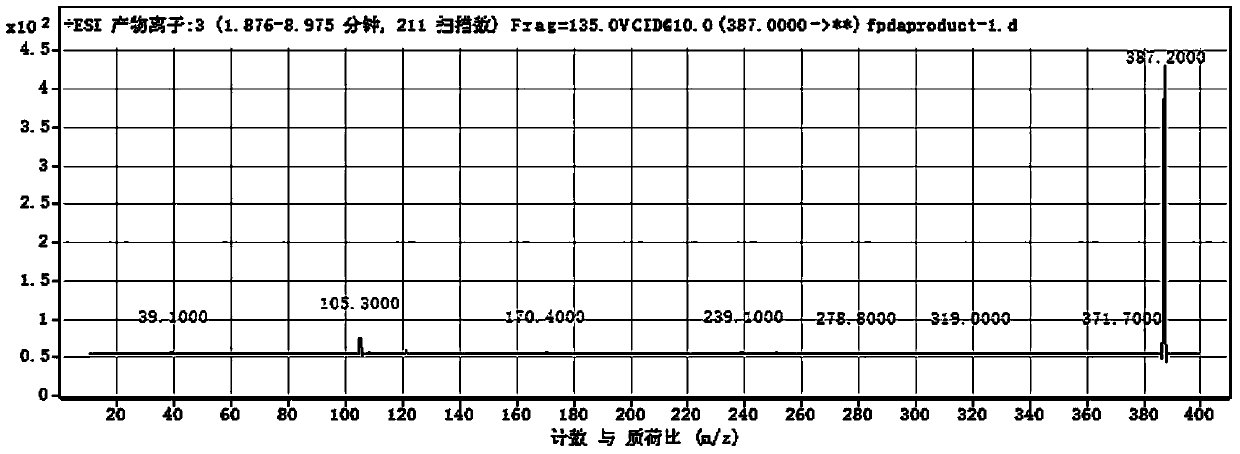
[0072] Wherein, R is N,N-dimethyl-1-naphthylamino, that is, the dopamine derivative is dansyl dopamine, and its molecular formula is C 20 h 22 N 2 SO 4 , the excitation wavelength is 340nm, and the emission wavelength is 514nm. The measurement result of its proton nuclear magnetic resonance spectrum shows that described dopamine derivative has following characteristic peak: 1 H NMR (400MHz): 8.51(d, J=8.6Hz, 1H), 8.19(d, J=5.8Hz, 1H), 8.14(d, J=7.2Hz, 1H), 7.53(t, J=8.0Hz ,1H),7.53(t,J=8.0Hz,1H),7.21(d,J=7.6Hz,1H),6.57(d,J=8.0Hz,1H),6.48(brs),6.29(br d, J=8.0Hz, 1H), 5.73(t, J=5.8Hz, 1H), 2.98(dt, J=5.8, 7.1Hz, 2H), 2.83(s, 6H), 2.44(t, J=7.1Hz, 2H). The liquid chromatography-mass spectrometry ESI detection of the dopamine derivative shows that its mass-to-charge ratio is 387.2 (molecular ion peak (M+H)+), and its molecular weight is 386. Dansyl dopamine has ...
Embodiment 2
[0075] A kind of dopamine derivative, is characterized in that, has the structure of formula (I):
[0076]
[0077] Wherein, R is a carbazole group, that is, the dopamine derivative is carbazolesulfonyl dopamine, and its molecular formula is C 20 h 18 N 2 SO 4 , has the structure of formula (III):
[0078]
PUM
| Property | Measurement | Unit |
|---|---|---|
| Wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com