Compound bis-spirophosphoryl nitrogen silane and preparation method thereof
A spirocyclic phosphorylazide silane and compound technology, applied in the field of compound double spirophosphorylazidesilane and its preparation, to achieve the effect of mild reaction conditions, high thermal stability, and improved thermal stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] The preparation method of compound double spirophosphoryl nitrogen silane, the steps are:
[0024] In the first step, dissolve 4.2mL (20.0mmol) hexamethyldisilazane in 10mL (20.0mmol) tetrahydrofuran at 0°C and nitrogen atmosphere, stir well and drop into 8mL (20.0mmol) n-butyl Lithium, after the dropwise addition, return to room temperature, and react for 1 hour; after the reaction, obtain a tetrahydrofuran solution of lithium hexamethyldisilazide, and set aside;
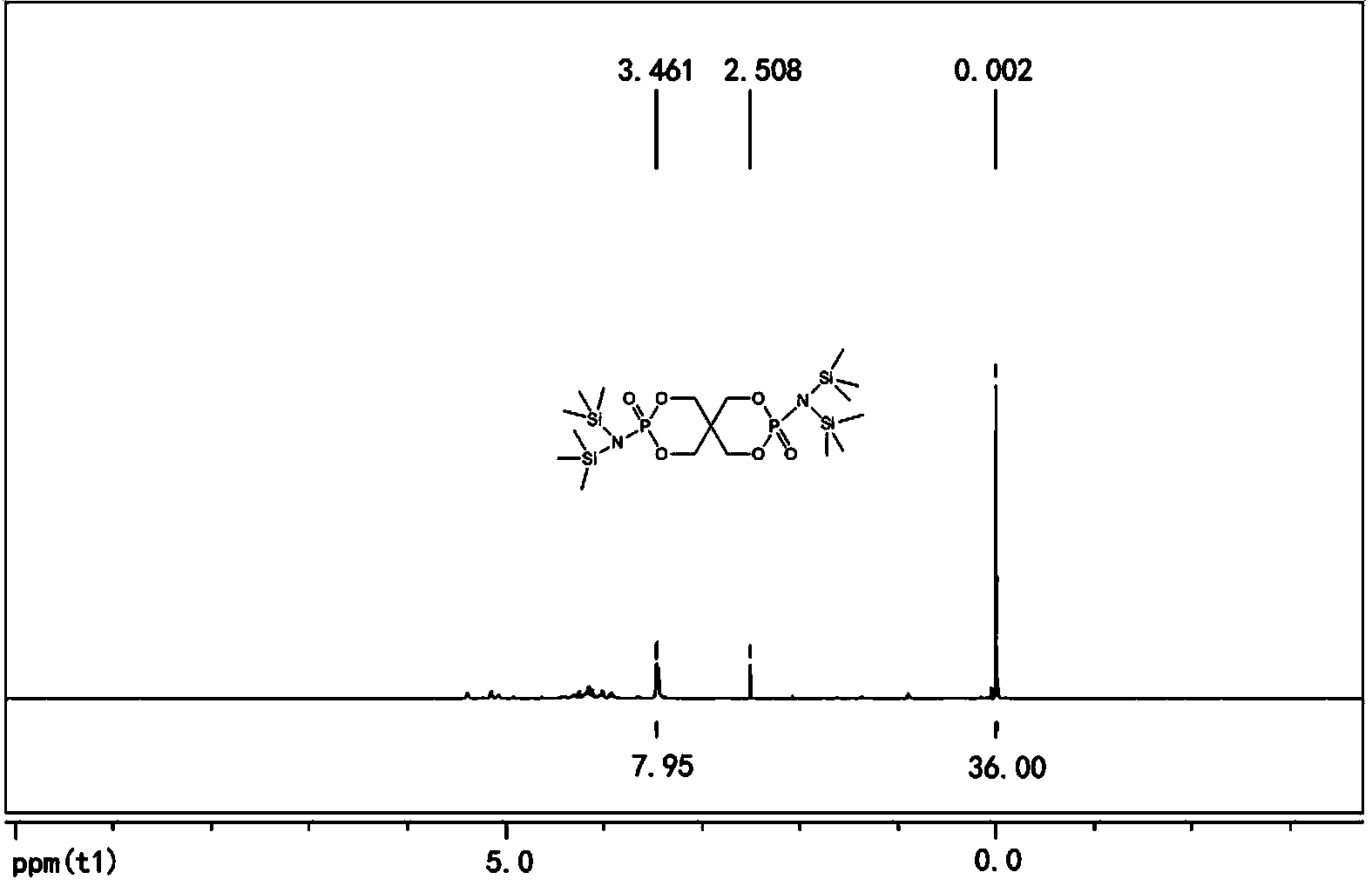
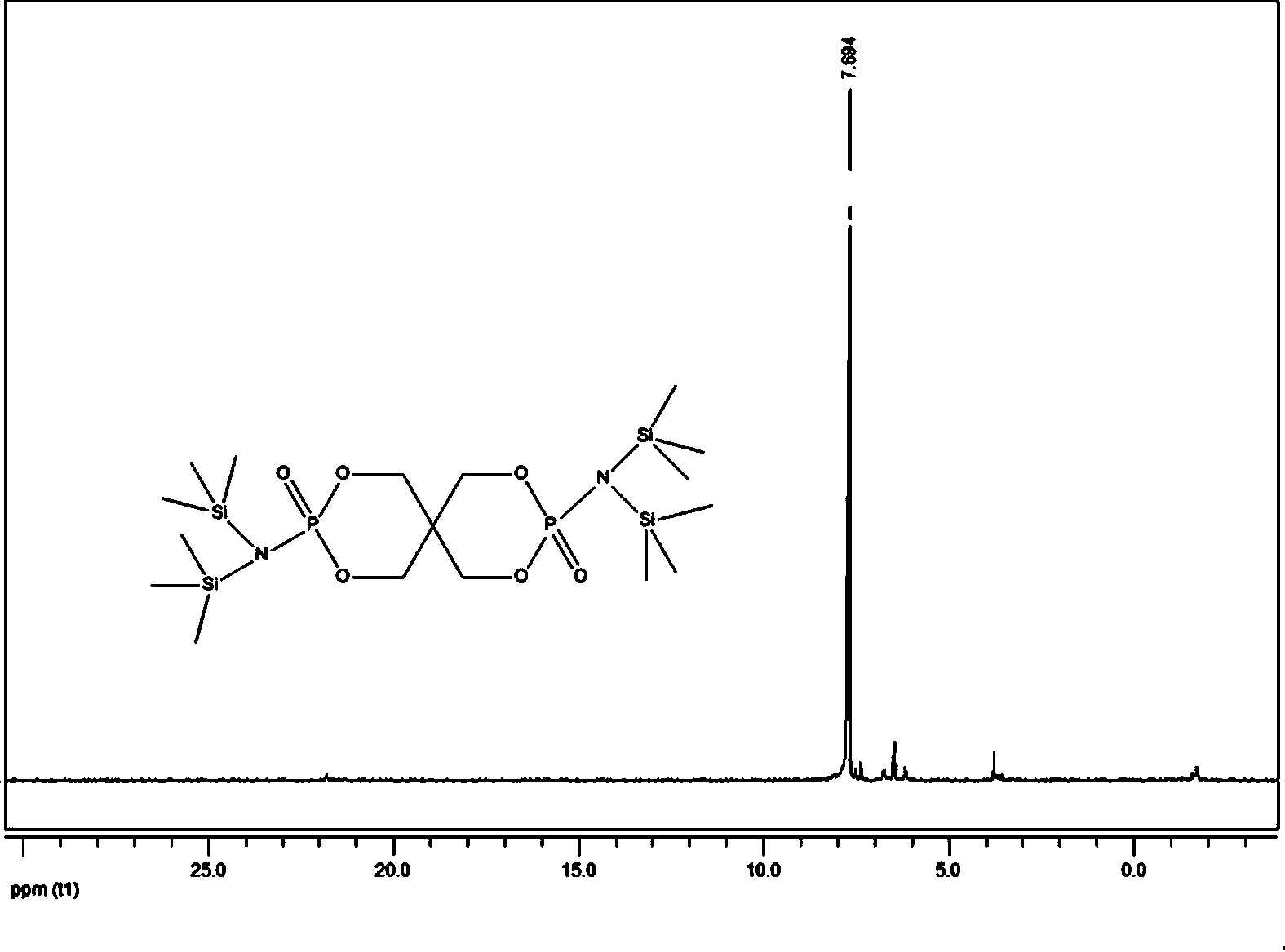
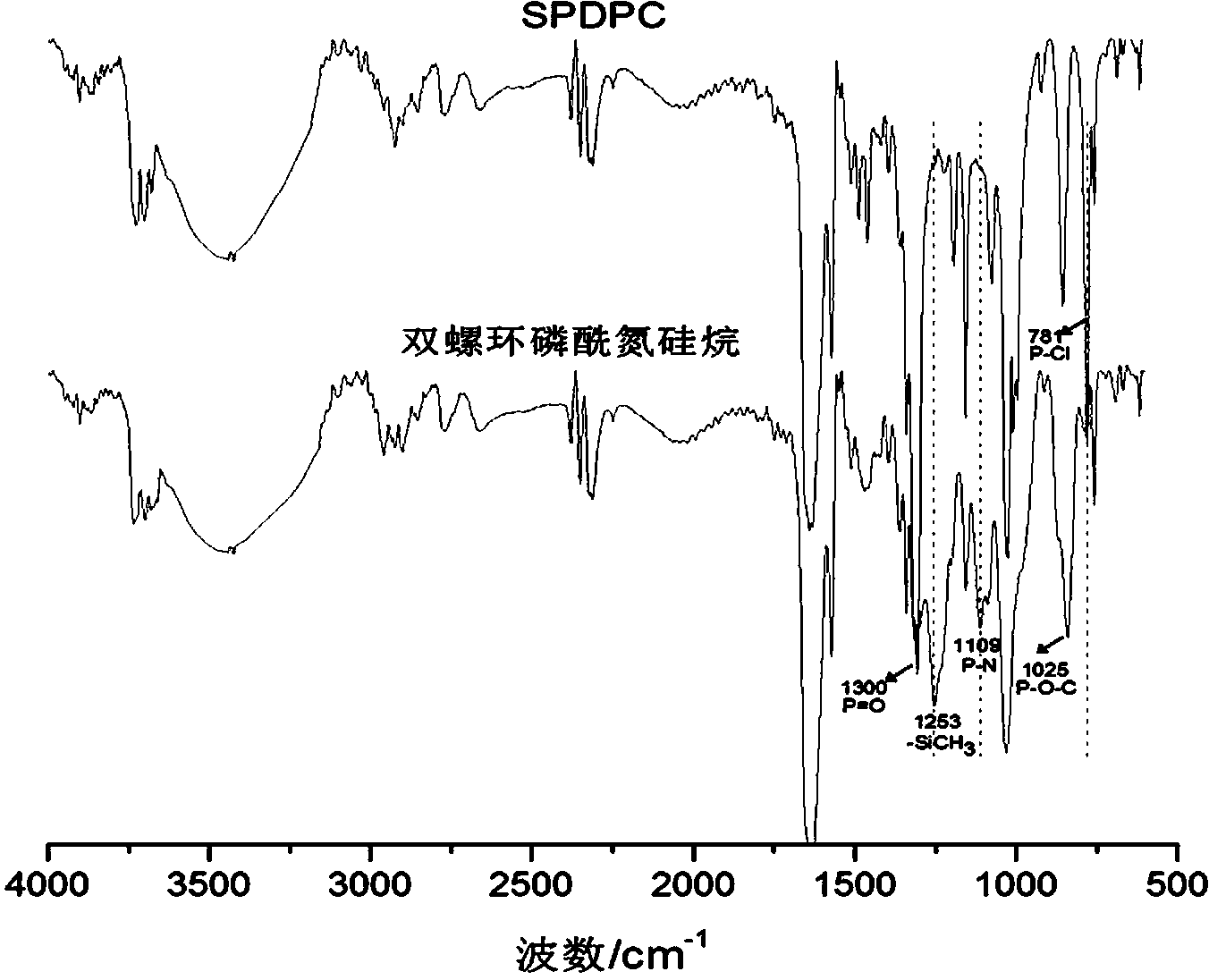
[0025] In the second step, add lithium hexamethyldisilazide tetrahydrofuran solution and 2.4g (8.0mmol) 3,9-dichloro-2,4, 8,10-tetraoxo-3,9-diphosphospirocyclo[5,5]-3,9-dioxundecane, reacted at -10°C for 6 hours, then returned to room temperature for 12 hours, filtered, The filtrate was rotary evaporated to obtain a solid primary product, which was washed three times with n-hexane and then once with anhydrous acetonitrile. After drying, 5.1 g of the compound bisspirophosphorylazidesilane was obtained, with ...
Embodiment 2
[0027] The preparation method of compound double spirophosphoryl nitrogen silane, the steps are:
[0028] The first step is to dissolve 8.4mL (40.0mmol) hexamethyldisilazane in 20mL (40.0mmol) tetrahydrofuran at 0°C and nitrogen atmosphere, stir well and drop into 16mL (40.0mmol) n-butyl Lithium, after the dropwise addition, return to room temperature, and react for 1.2h; after the reaction, obtain a tetrahydrofuran solution of lithium hexamethyldisilazide, and set aside;
[0029] In the second step, add lithium hexamethyldisilazide tetrahydrofuran solution and 5.3 g (18.0 mmol) 3,9-dichloro-2,4, 8,10-tetraoxo-3,9-diphosphospirocyclo[5,5]-3,9-dioxundecane, reacted at 0°C for 1 hour, then returned to room temperature for 12 hours, filtered, and the filtrate The solid primary product was obtained by rotary evaporation, which was washed twice with n-hexane and then once with anhydrous acetonitrile. After drying, 8.7 g of the compound bisspirophosphorylazidesilane was obtained, w...
Embodiment 3
[0031] The preparation method of compound double spirophosphoryl nitrogen silane, the steps are:
[0032] The first step is to dissolve 16.8mL (80.0mmol) hexamethyldisilazane in 40mL (80.0mmol) tetrahydrofuran at 0°C and nitrogen atmosphere, stir well and drop into 32mL (80.0mmol) n-butyl Lithium, after the dropwise addition, return to room temperature, and react for 1.5h; after the reaction, obtain a tetrahydrofuran solution of lithium hexamethyldisilazide, and set aside;
[0033] In the second step, add lithium hexamethyldisilazide tetrahydrofuran solution and 10.0 g (33.3 mmol) 3,9-dichloro-2,4, 8,10-tetraoxo-3,9-diphosphospirocyclo[5,5]-3,9-dioxundecane, reacted at -20°C for 3 hours, then returned to room temperature for 12 hours, filtered, The filtrate was rotary evaporated to obtain a solid primary product, which was washed three times with n-hexane and then once with anhydrous acetonitrile. After drying, 16.0 g of the compound bisspirophosphorylazidesilane was obtained...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com