Bis ether compounds having fluorene skeleton and resin composition
A compound and composition technology, applied in the field of aromatic bishalogenated methyl compounds, can solve the problems of high refractive index and insufficient dimensional stability of optical lenses, and achieve small dielectric loss tangent, high heat resistance, The effect of excellent optical characteristics
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0137] Add 140.16g (0.40 mol) of 9,9-bis(4-hydroxyphenyl)fluorene, 141.40g (0.88 mol) of 4,4'-bis(chloromethyl)biphenyl and 1200ml of acetone into the reaction vessel, stirring While raising the temperature to 78°C. Next, KOH-MeOH (KOH: 0.88 mol) was dropped into the reaction vessel kept at 78° C. over 30 minutes. After completion of the dropping, stirring was further continued at 78° C. for 4 hours. After 4 hours, it was cooled to room temperature, 900 ml of toluene was added, and 10% HCl was further added for neutralization. Then, the aqueous phase was separated by liquid separation, and further washed three times with 300 ml of water.
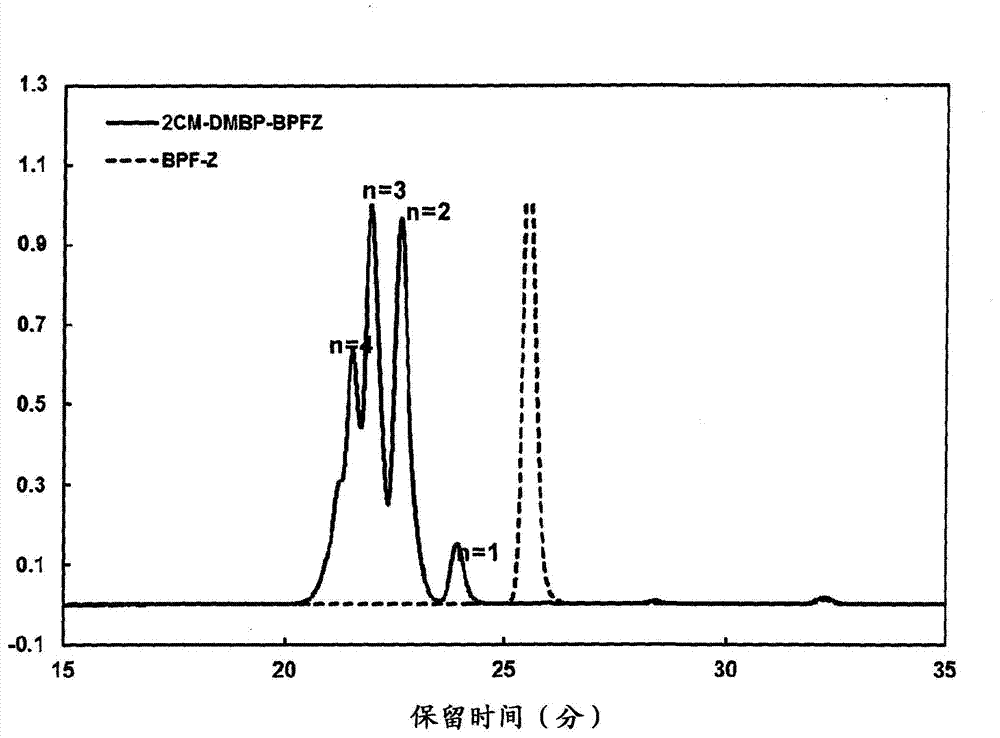
[0138] The obtained organic phase was concentrated by distillation, methanol was added, and the product was reprecipitated. The precipitate was filtered and dried to obtain the aromatic bischloromethyl compound A which is a reaction product of 9,9-bis(4-hydroxyphenyl)fluorene and 4,4'-bis(chloromethyl)biphenyl (2CM-DMBP-BPFZ) 169.33g. T...
Embodiment 2
[0151] Add 140.16g (0.40 mole) of 9,9-bis(4-hydroxyphenyl)fluorene, 157.20g (0.88 mole) of α,α'-dichloro-p-xylene and 1200ml MEK into the reaction vessel, and heat up while stirring to 78°C. Next, KOH-MeOH (KOH: 0.88 mol) was dripped into the reaction container maintained at 78 degreeC over 30 minutes. After completion of the dropping, stirring was further continued at 78° C. for 4 hours. After 4 hours, it was cooled to room temperature, 900 ml of toluene was added, and 10% HCl was further added for neutralization. Then, the aqueous phase was separated by liquid separation, and further liquid separation washing was performed three times with 300 ml of water.
[0152] The obtained organic phase was concentrated by distillation, methanol was added, and the product was reprecipitated.
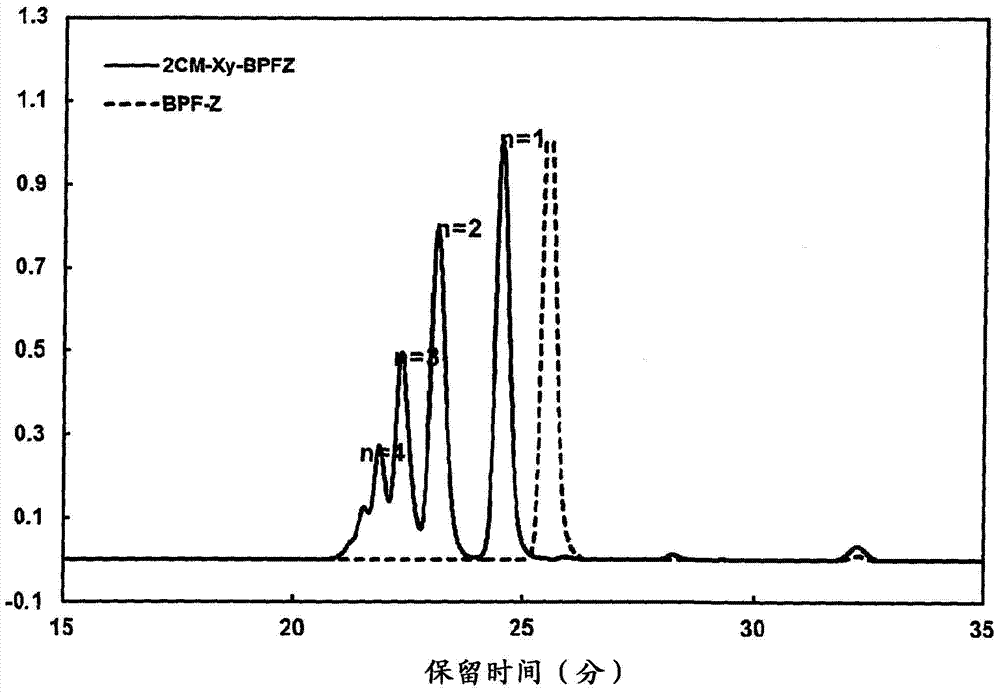
[0153] The precipitate was filtered and dried to obtain the aromatic bischloromethyl compound B ( 2CM-Xy-BPFZ) 123.57g.
[0154] Gel permeation chromatography (GPC), infrared spectroscopy (IR...
Embodiment 3
[0165] 15.28 g (0.11 mol) of potassium carbonate and 500 ml of N,N-dimethylformamide (DMF) were placed in a reaction vessel, and heated and stirred. After the internal temperature of the reaction container reached 80° C., a solution obtained by dissolving 19.13 g (0.22 mol) of methacrylic acid in 50 ml of DMF was dropped over 30 minutes. Maintaining this temperature, the reaction was carried out for 1 hour. Next, a solution obtained by dissolving 58.27 g of 2CM-DMBP-BPFZ obtained in Example 1 in 500 ml of DMF was added dropwise over 30 minutes. After completion of the dropping, stirring was further continued at 80° C. for 3 hours. After 3 hours, it was cooled to room temperature, and the precipitated solid was separated by filtration. Then, 2000 ml of toluene was added to the reaction solution. Then, the reaction solution was washed with water four times, and the oily phase was dried over magnesium sulfate and filtered. The obtained organic phase was reprecipitated with a ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| turbidity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com