Gynostemma pentaphylla saponin long-circulating liposome preparation and preparation method thereof
A gypenoside and liposome technology, which is applied in liposome delivery, pharmaceutical formulations, medical preparations of non-active ingredients, etc., can solve the problems of no gypenoside long-circulation liposome reports, and achieve steric stabilization The effect of long circulation in the body, reducing the probability of cardiovascular and cerebrovascular diseases, and enhancing the stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
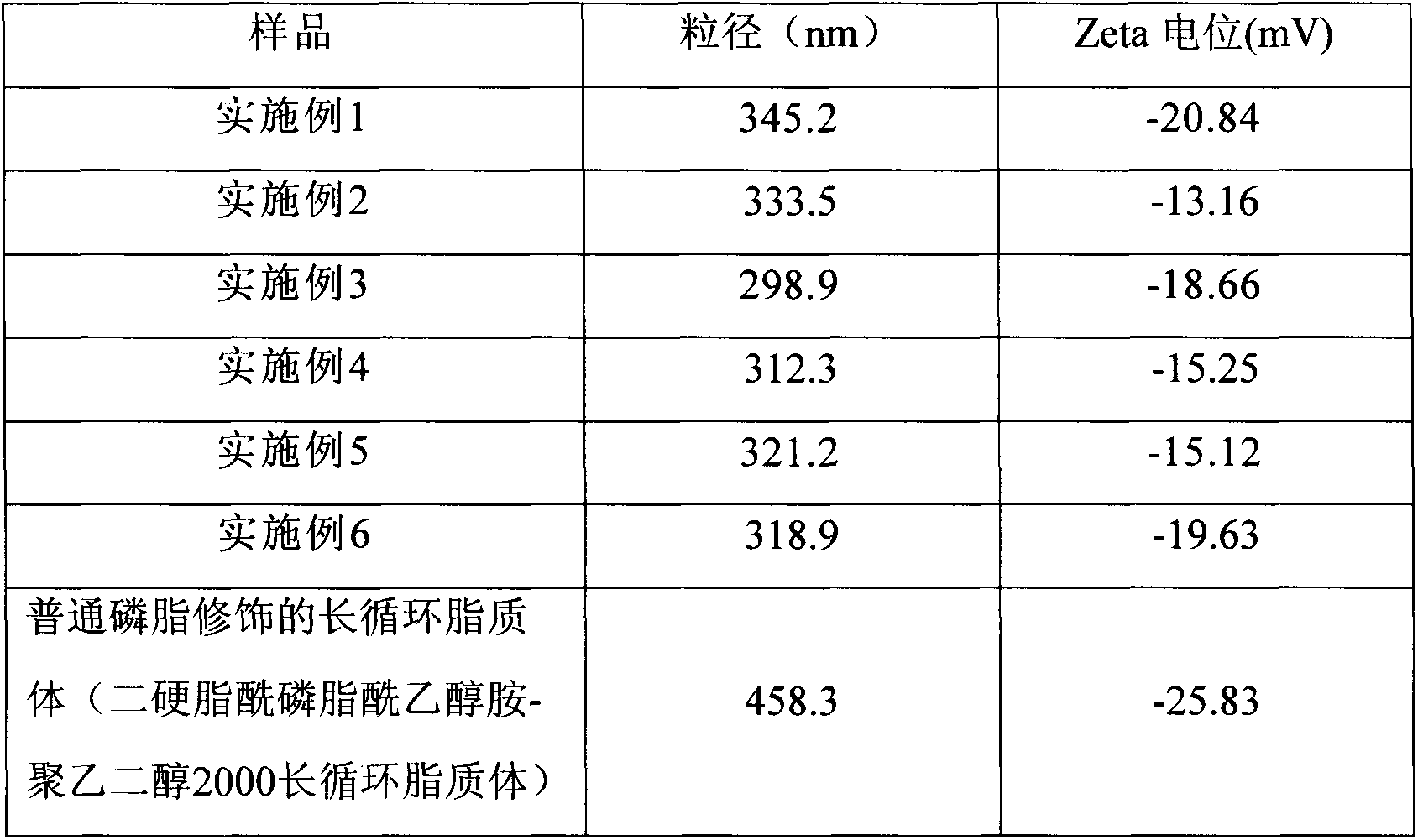
Embodiment l
[0038] prescription Dosage Distearoylphosphatidylethanolamine (DSPE) 200mg Compound of formula I 100mg Ether 3mL Gypenoside 20mg Phosphate buffer (pH=7.0) 15mL
[0039] craft
[0040] (1) selection of raw materials: get phosphate buffer saline and gypenoside by above-mentioned prescription, gypenoside is dissolved in phosphate buffer saline, for subsequent use;
[0041] (2) preparation of gypenoside liposome: get distearoylphosphatidylethanolamine, formula I compound and ether by above-mentioned prescription, mix homogeneously, then rotary evaporation makes it become uniform lipid film and covers the bottom in the pear-shaped bottle, Add gypenoside-containing phosphate buffer solution to the bottle, add glass beads, rotate and shake at 60°C for 30 minutes, and place it for 2 hours to fully hydrate.
Embodiment 2
[0043] prescription Dosage Gypenoside 20mg Lecithin 200mg Compound of formula I 50mg ethanol 10mL Phosphate buffer (pH=6.8) 10mL
[0044] craft
[0045] (1) Preparation of oil phase: get gypenoside, lecithin, formula I compound, ethanol according to the above prescription, mix well, as oil phase;
[0046] (2) Preparation of the water phase: take phosphate buffer as the water phase according to the above prescription;
[0047] (3) Preparation of liposomes: under constant temperature stirring, inject the oil phase into the water phase at a temperature of 45°C at a constant speed with a needle, stir at constant temperature for 2 hours to remove ethanol, and obtain the liposome.
Embodiment 3
[0049] prescription Dosage Gypenoside 200mg Phosphatidylserine 200mg Compound of formula I 50mg Dichloromethane 50mL Phosphate buffer (pH=8.0) 40mL
[0050] craft
[0051] (1) Take phosphatidylserine according to the above prescription: compound of formula I: dichloromethane=200mg: 50mg: 50mL to prepare the oil phase, place the oil phase in a round-bottomed flask, and rotate to evaporate the dichloromethane;
[0052] (2) 200mg gypenoside was dissolved in 20mL phosphate buffer (pH=8.0) as the water phase;
[0053] (3) Add 20 mL of phosphate buffer solution (pH=8.0) containing 200 mg of gypenoside to the oil phase, and in an ice bath, sonicate the probe for 10 minutes to form a uniform emulsion;
[0054] (4) Rotary evaporation to remove the organic phase, after forming a colloidal state, continue to add 20 mL of phosphate buffer, continue to evaporate under reduced pressure for 0.5 h, and ultrasonically probe for 10 min to obtai...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com