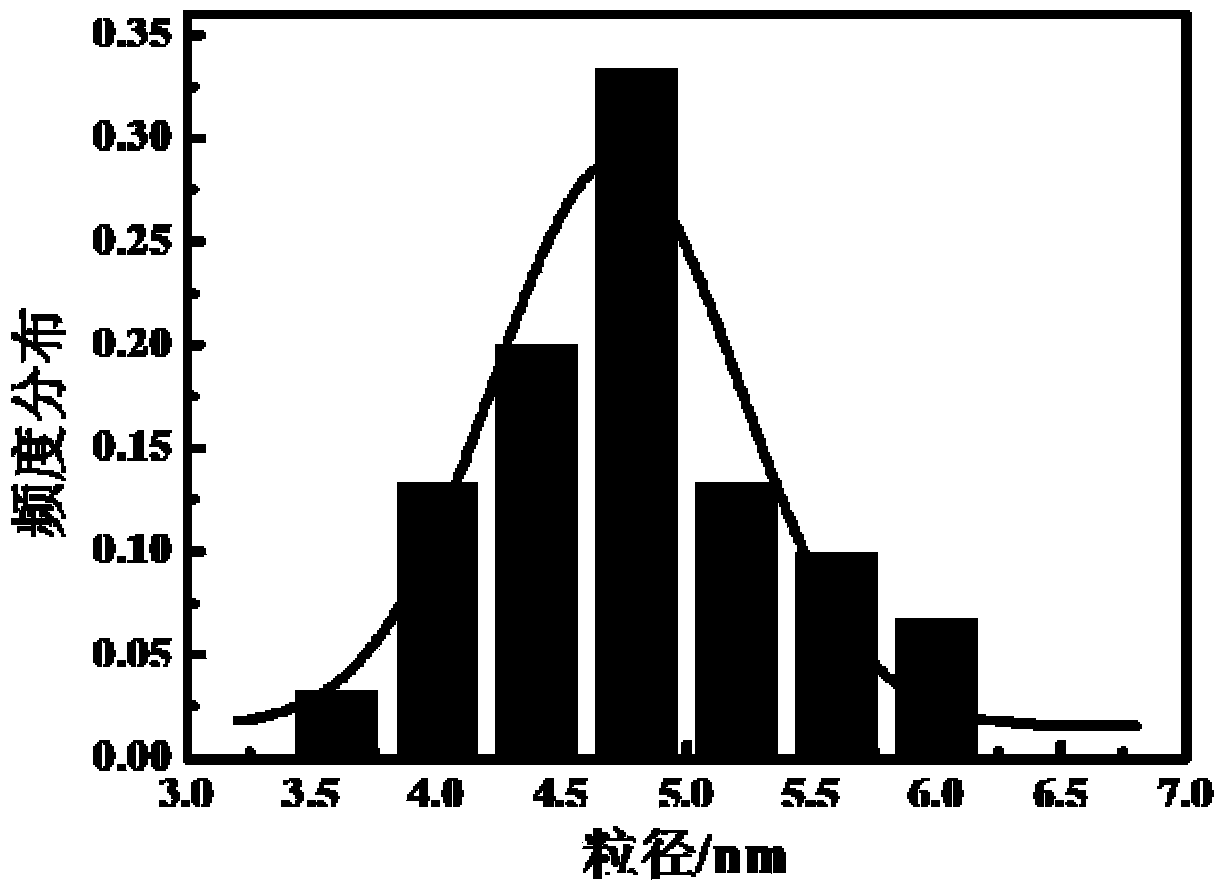
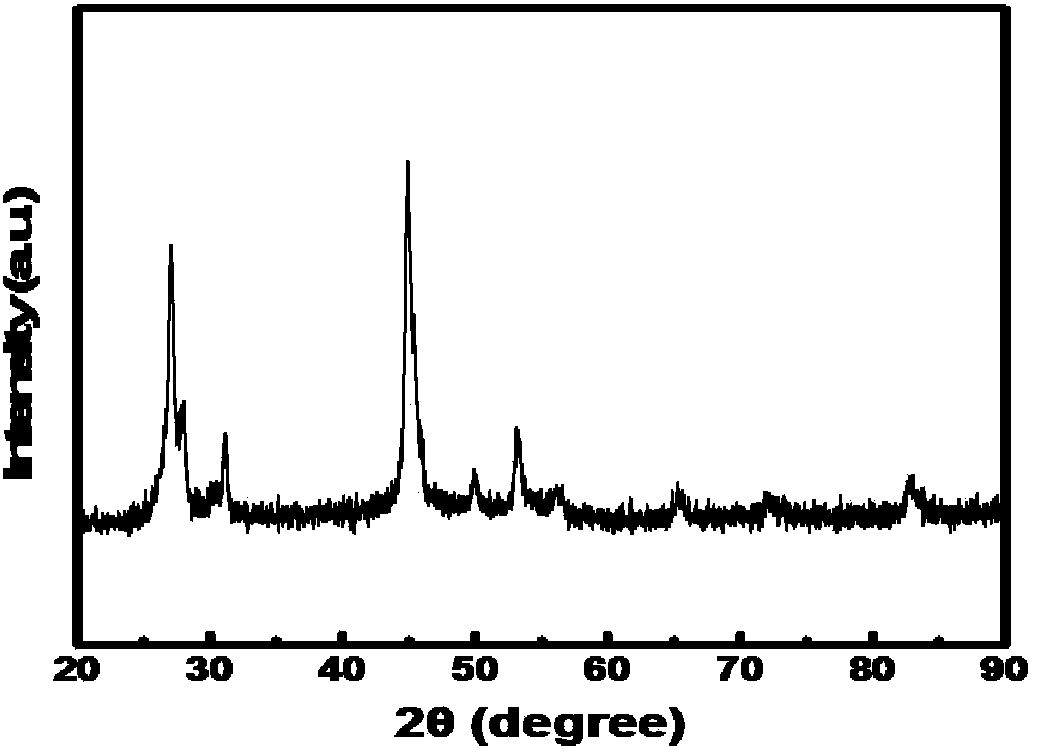
Copper selenide fluorescent quantum dot and preparation method and application thereof
A technology of fluorescent quantum dots and copper selenide, applied in chemical instruments and methods, photosensitive equipment, binary selenium/tellurium compounds, etc., can solve problems such as retention, expensive raw materials, limited expansion and perfection, etc., and achieve mild conditions , good fluorescence stability and high luminous efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] 1. Preparation of Reaction Precursor
[0037] Weigh 0.1mmol of CuCl dissolved in 25mL of deionized water, then add 0.1mmol of oleic acid in 50mL of chloroform solution into the system, stir magnetically at 100rmp to form a water-oil reaction system, and then heat to 30°C for 1 hour under nitrogen gas to obtain the reaction precursor body solution.
[0038] 2. Cu 2 Preparation of Se quantum dots
[0039] Weigh 0.1mmol (0.0038g) NaBH 4 and 0.05mmol (0.004g) of Se powder, dissolved in 1g of pure water and reacted in an ice-water bath for about 3 hours to obtain a purple-red NaHSe solution, inject the reaction precursor solution under a hot nitrogen atmosphere, stir magnetically, and the stirring speed is 100rmp, The reaction was stopped after 1 h. Take the upper layer of chloroform phase solution and precipitate it with a large amount of ethanol, and then disperse it in toluene after centrifuging at a speed of 15,000 rpm for 30 minutes. After repeated repetitions, a bl...
Embodiment 2
[0043] 1. Preparation of Reaction Precursor
[0044] Weigh 1 mmol (0.170 g) of CuCl2 and dissolve it in 50 mL of deionized water, then add 25 mL of n-hexane solution dissolved with 10 mmol of undecylenic acid into the system, stir magnetically at 300 rpm to form a water-oil reaction system, and heat to 90 °C to obtain a reaction precursor solution.
[0045] 2. Preparation of CuSe quantum dots
[0046] Weigh 4mmol (0.1513g) NaBH 4 and 2mmol (0.1579g) of Se powder, dissolved in 1g of pure water, and reacted in an ice bath for about 5 hours to obtain a purple-red NaHSe solution, then injected into the reaction precursor solution under a hot nitrogen atmosphere, magnetically stirred, and the stirring speed was 300rmp, 8h After stopping the reaction. The upper n-hexane phase solution was precipitated with a large amount of ethanol, centrifuged at 15,000 rpm for 30 minutes, and then dispersed in toluene. After repeated repetitions, a blue fluorescent CuSe quantum dot solution was...
Embodiment 3
[0050] 1. Preparation of Reaction Precursor
[0051] Weigh 1mmol (0.242g) CuNO 3 After dissolving in 50mL deionized water, add 50mL n-hexane solution dissolved in 10mmol stearic acid into the system, stir magnetically at 300rmp to form a water-oil reaction system, and heat to 70°C after nitrogen gas for 1h to obtain a reaction precursor solution.
[0052] 2. Preparation of CuSe quantum dots
[0053] Weigh 2mmol (0.076g) NaBH 4 and 1mmol (0.079g) of Se powder, dissolved in 1g of pure water and reacted in an ice bath for about 5 hours to obtain a dark purple NaHSe solution, then injected into the reaction precursor solution under a hot nitrogen atmosphere, magnetically stirred at a stirring speed of 300rmp, 9h After stopping the reaction. Take the upper n-hexane phase solution and precipitate it with a large amount of ethanol, centrifuge at 12000rpm for 20min, and then disperse it in toluene. After repeated repetitions, a blue fluorescent CuSe quantum dot solution is obtained...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| full width at half maximum | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com