Combined drug-loading micelle of targeted integrin receptor and preparation method thereof
An integrin receptor, drug-loaded micelle technology, which is applied in pharmaceutical formulations, medical preparations with inactive ingredients, and medical preparations containing active ingredients, can solve the problems of low drug loading in the micelle system, and achieve The effect of increasing drug loading and improving therapeutic index
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Example 1 Preparation of amphiphilic block copolymer Pluronic-COOH with active group carboxyl
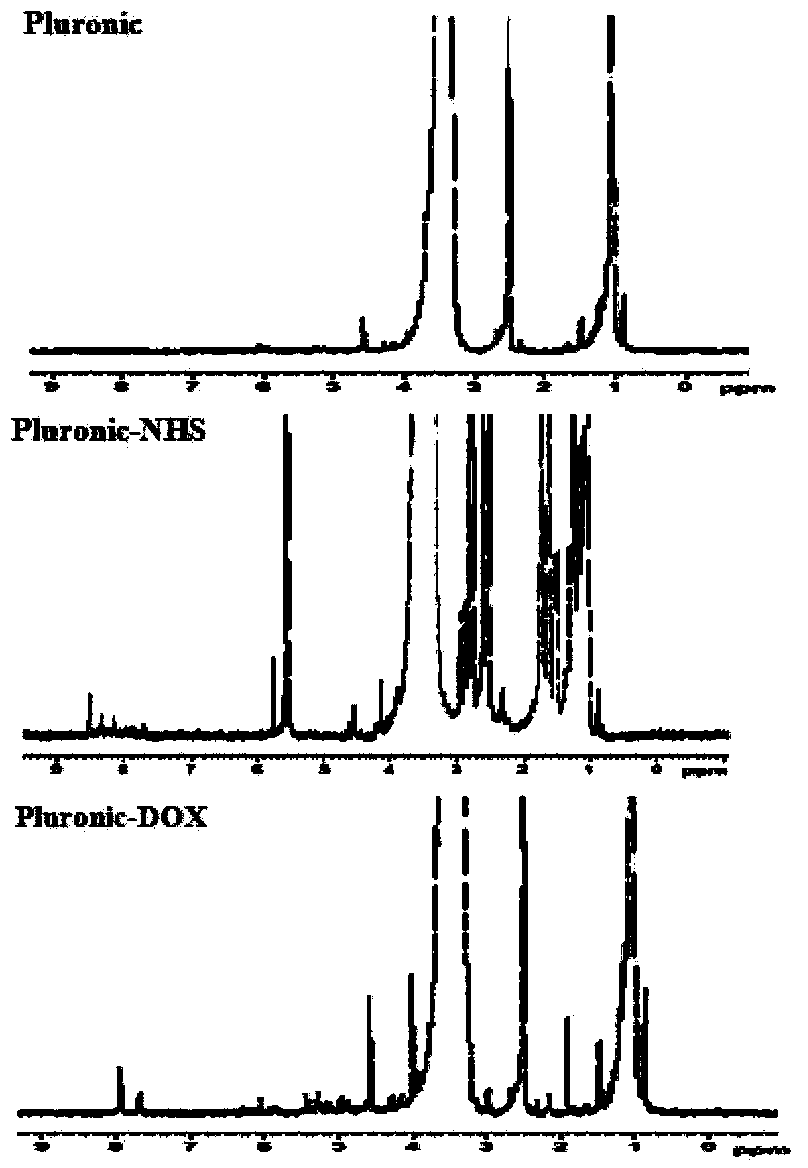
[0042] Put 0.01M Pluronic and 400ml acetone in a round-bottomed flask, slowly heat up until the solution is clear, let cool to room temperature, add 17ml Jones reagent, magnetically stir overnight at room temperature, add 5mL isopropanol to quench the reaction. Add 12.6g of activated carbon and continue to stir for 2h, heat to 40°C, suction filter while hot to obtain a clear solution, evaporate under reduced pressure to obtain a white viscous substance, add precooled n-hexane to precipitate a solid, and dry in vacuo to obtain carboxylated Pluronic ( Pluronic-COOH). The purity of the product was analyzed by gel permeation chromatography (GPC). 1 The molecular weight of the product was calculated from the peak area ratio of the proton in the methylene group in the H-NMR spectrum.
Embodiment 2
[0043] Embodiment 2 prepares Pluronic-DOX
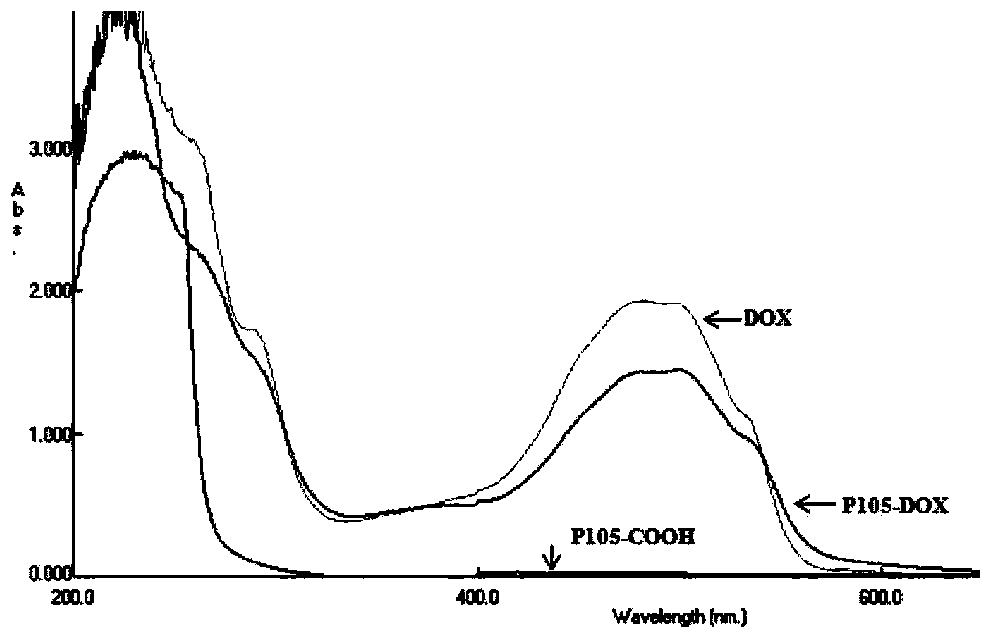
[0044] Weigh 7.4mM of Pluronic-COOH in the above Example 1, and dissolve it in 20ml of N,N-dimethylformamide at room temperature. 1 g EDC and 2 g NHS were added to the solution of Pluronic-COOH. After reacting for 15 minutes, 2.8ml of 2-mercaptoethanol, 3.48g of DOX and 10mg of triethylamine were added, and stirring was continued at room temperature for 12h under nitrogen protection. After the reaction, dialyze with deionized water in the dark for 2 days (MWCO 3500), and freeze-dry the concentrated solution to obtain Pluronic-DOX. pass 1 The product was verified by H-NMR spectrum; the absorbance was measured by UV-Vis spectrophotometry, and the molar connection rate of DOX was calculated.
Embodiment 3
[0045] Embodiment 3 prepares the amphiphilic copolymer modified with RGD peptide
[0046] (1) Preparation of Pluronic-NHS by activating the carboxyl group with NHS:
[0047] Weigh 0.053mM of Pluronic-COOH, 22mg of DCC and 12.2mg of NHS in the above-mentioned Example 1 and dissolve them in 5ml of chloroform, and react at room temperature for 24h under nitrogen protection. After the reaction was completed, the solvent was removed under reduced pressure and precipitated with cold ether. The precipitate was vacuum-dried to constant weight to obtain Pluronic-NHS. The product is verified by 1H-NMR spectrum;
[0048] (2) Preparation of c(RGDyK)-Pluronic by cyclic RGD peptide (c(RGDyK)) modified amphiphilic copolymer;
[0049] Take the Pluronic-COOH 0.0053mM in the above (1) and dissolve it in 1ml of N,N-dimethylformamide to obtain solution A; take 6.3mg (0.01mM) of c(RGDyK) peptide and dissolve it in 0.1M HEPES, Solution B is obtained. Solution B was added dropwise to solution A...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com