Protein antimicrobial catheter and manufacturing method thereof
A urinary catheter and protein technology, applied in catheters, coatings and other directions, can solve the problems of complex surface coating treatment process, weak persistence of antibiotics, affecting bacteriostatic and antibacterial efficacy, etc. The effect of reducing treatment time and reducing physical pain
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
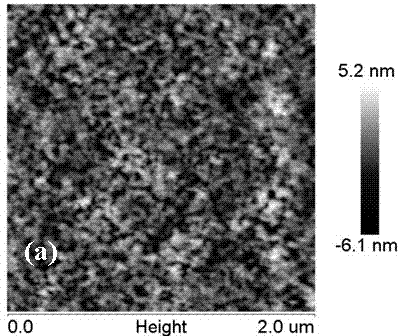
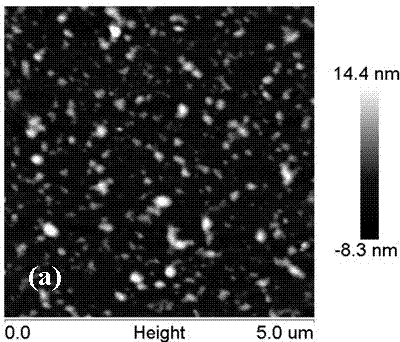
[0039] Further in the preparation method of the above-mentioned protein antibacterial catheter, the natural protein is serum albumin, silk fibroin, whey protein, collagen, walbumin, ovalbumin, soybean albumin, lysozyme One or more; the solvent is an aqueous solution of water or ethanol, ethylene glycol, glycerin, mannitol, sodium chloride, calcium chloride, sodium bicarbonate; the non-protein natural substance is chitin, chitosan Sugar or its derivatives are added in an amount of 1% to 5% of the total weight of the initial glue; the cross-linking agent is one or more of carbodiimide, N-hydroxysuccinimide or genipin Combination, its addition is 0.5%~1.5% of the initial glue total weight; The thickener is one or more combinations of sodium carboxymethyl cellulose, xanthan gum or sodium alginate, and its addition is 0.5% to 1.5% of the total weight of the initial glue; the dispersant is one or more combinations of sodium tripolyphosphate, polyethylene glycol 400 or Tween 60, and ...
Embodiment 1
[0042] Protein antibacterial catheter, the preparation process is as follows:
[0043] Catheter pretreatment: Take a complete silicone rubber catheter raw material, wash it repeatedly with distilled water 5 to 6 times, and then completely immerse it in Piranha lotion (98% concentrated sulfuric acid and 30% hydrogen peroxide solution 3:1 (v / v) mixed solution) in a water bath at 60°C for 30 min.
[0044] Preparation of protein glue: mix soluble powders of whey protein, wheat albumin, lysozyme, and carboxymethyl chitosan in a certain mass ratio, such as 1.5:5:3:0.5, and add them into distilled water to obtain the total mass fraction 2wt% to 20wt% of the initial glue, then according to the volume of the protein glue, add 5mg / ml polyethylene glycol 400 aqueous solution in a ratio of 50:1 (v / v) as a dispersant, and use a magnetic stirrer for the solution Stir for 1 hour to obtain a secondary glue, and finally filter and centrifuge the secondary glue successively to remove air bubbl...
Embodiment 2
[0048] Protein antibacterial catheter, the preparation process is as follows:
[0049] Pretreatment of urinary catheter: Take a complete latex urinary catheter, wash it repeatedly with 80% (v / v) ethanol aqueous solution for 5 to 6 times, let the catheter dry naturally, and then use ethylene oxide to dry it. It was sterilized at 50° C. for 10 minutes, and then placed in a continuous radio frequency low-temperature plasma apparatus to treat the surface of the catheter. The plasma discharge power and time were 200 W and 5 minutes, respectively.
[0050] Protein glue preparation: mix the soluble powders of silk fibroin, whey protein, wheat albumin and ovalbumin according to a certain mass ratio, such as 1:4:3:2, and add 20% (v / v) Ethylene glycol solution is used as a solvent to obtain an initial glue solution with a total mass fraction of 5wt% to 20wt%, and then 0.01mol / L sodium tripolyphosphate solution is added in a ratio of 50:1 (v / v) according to the volume of the protein glue...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| surface roughness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com