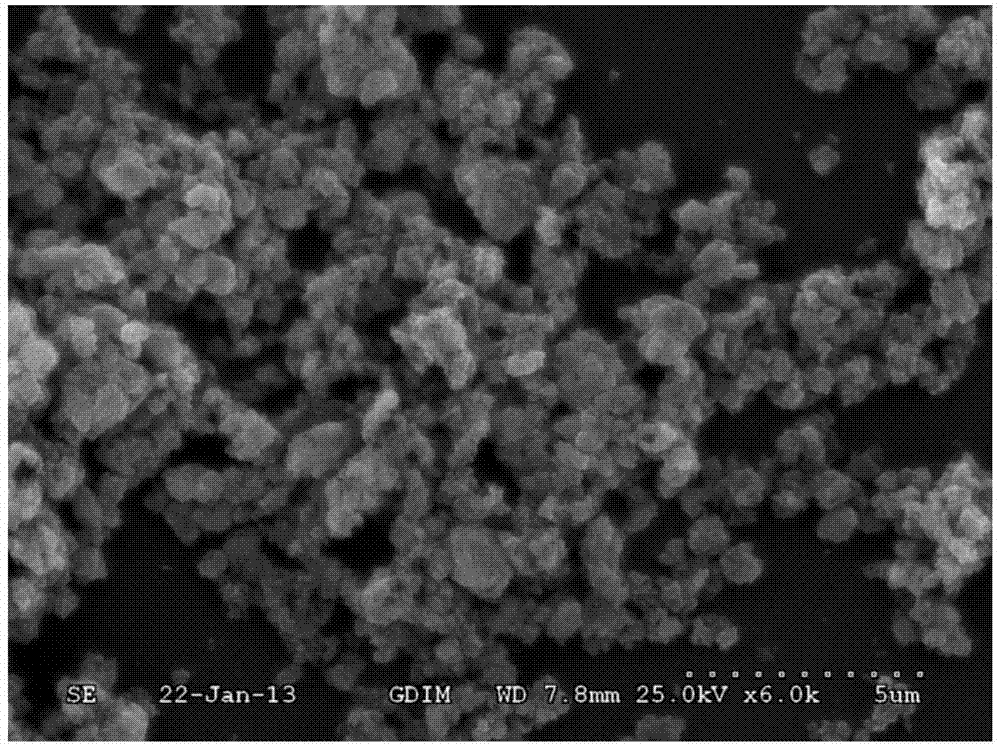
Novel strontium-doped gamma-polyglutamic acid/tricalcium phosphate composite material and preparation method thereof
A polyglutamic acid and tricalcium phosphate technology, applied in the field of biomedicine, can solve the problems of high brittleness, limited depth of bone ingrowth, low flexural strength, etc., and achieve improved mechanical properties, good biocompatibility, and no cells. toxic effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] According to the amount of calcium ions and phosphorus ions in the chemical formula of tricalcium phosphate, that is, the molar ratio of Ca:P=3:2, calcium chloride, sodium dihydrogen phosphate and strontium chloride hexahydrate were selected to prepare the same volume concentration as 0.3mol / L calcium ion solution, phosphorus ion solution with a concentration of 0.2mol / L and strontium ion solution with a concentration of 0.1mol / L. Mix equal volumes of calcium ion solution and strontium ion solution. The mass fraction is 2.0% gamma-polyglutamic acid sodium salt solution, and the molar ratio of carboxyl and calcium ion in the gamma-polyglutamic acid sodium salt solution is 6:1, and gamma-polyglutamic acid sodium The salt solution is added to the mixed solution of the calcium ion solution and the strontium ion solution, and the reaction is stirred at 50° C. for 5 hours. In the reaction solution of γ-polyglutamic acid sodium salt and calcium and strontium ions, add the ab...
Embodiment 2
[0021] According to the amount of calcium ions and phosphorus ions in the chemical formula of tricalcium phosphate, that is, the molar ratio of Ca:P=3:2, calcium nitrate tetrahydrate, potassium dihydrogen phosphate and strontium nitrate are selected, and the concentration of the same volume is 6mol / L. Calcium ion solution with a concentration of 4mol / L phosphorus ion solution and strontium ion solution with a concentration of 0.15mol / L. Mix equal volumes of calcium ion solution and strontium ion solution. The mass fraction is 2.5% gamma-polymethyl glutamate gel liquid, and the molar ratio of carboxyl and calcium ions in the gamma-poly glutamate gel liquid is 2:1, and the gamma-poly The methyl glutamate gel solution was added to the mixed solution of the calcium ion solution and the strontium ion solution, and stirred and reacted at 60° C. for 4 hours. In the reaction solution of γ-polymethyl glutamate and calcium and strontium ions, add the above-mentioned phosphorus ion solu...
Embodiment 3
[0023] According to the amount of calcium ions and phosphorus ions in the chemical formula of tricalcium phosphate, that is, the molar ratio of Ca:P=3:2, choose calcium nitrate tetrahydrate, disodium hydrogen phosphate and strontium nitrate, and prepare the same volume concentration of 60mol / L Calcium ion solution with a concentration of 40mol / L phosphorus ion solution and strontium ion solution with a concentration of 0.5mol / L. Mix equal volumes of calcium ion solution and strontium ion solution. The mass fraction is 4% γ-polyglutamic acid polysaccharide composite gel liquid, and the molar ratio of the carboxyl group in the γ-polyglutamic acid polysaccharide polysaccharide composite gel liquid is 10:1, and the γ-polysaccharide The glutamic acid-polysaccharide composite gel solution is added to the mixed solution of the calcium ion solution and the strontium ion solution, and stirred and reacted at 80° C. for 2 hours. In the reaction solution of γ-polyglutamic acid polysaccha...
PUM
| Property | Measurement | Unit |
|---|---|---|
| compressive strength | aaaaa | aaaaa |
| flexural strength | aaaaa | aaaaa |
| compressive strength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com