Method for producing electrolytic manganese metal
A technology of electrolytic manganese metal and electrolyte, which is applied in the direction of photographic process, instrument, photographic auxiliary process, etc., can solve the problems of increasing the burden of solution purification, manganese ore cannot be effectively utilized, and the production of manganese alloy products, etc. effect, prevent impurities from entering the solution, and improve quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
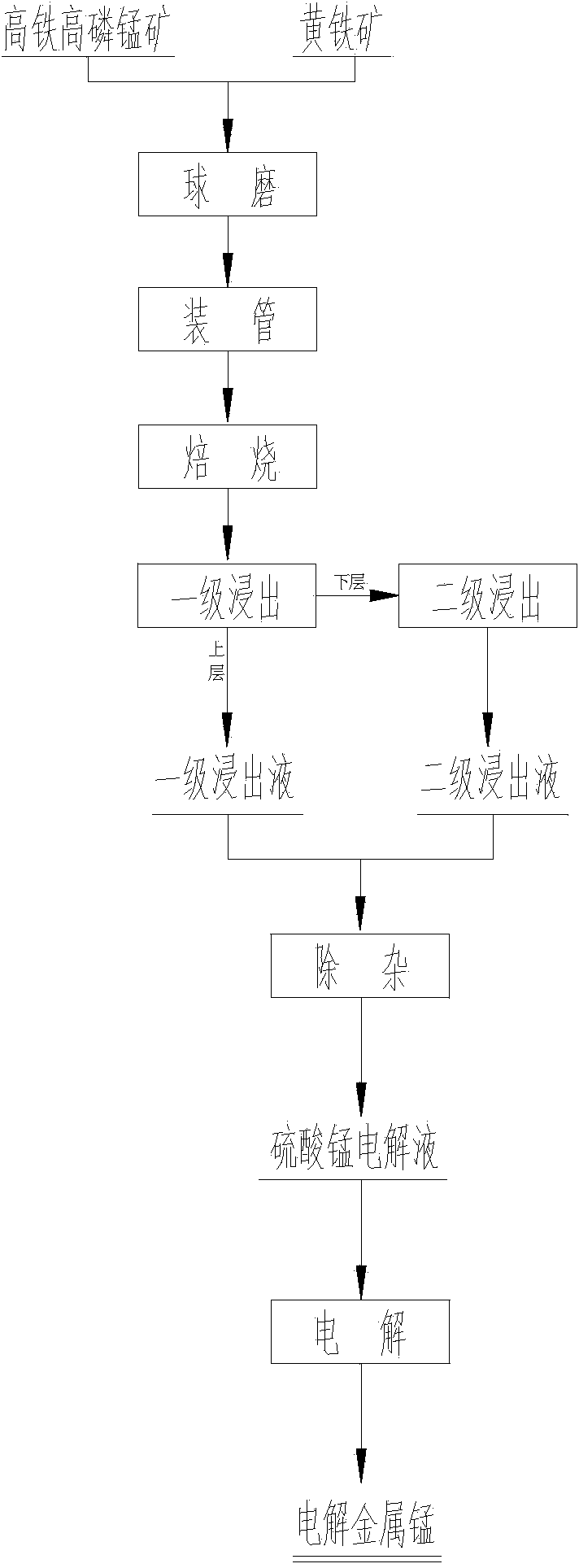
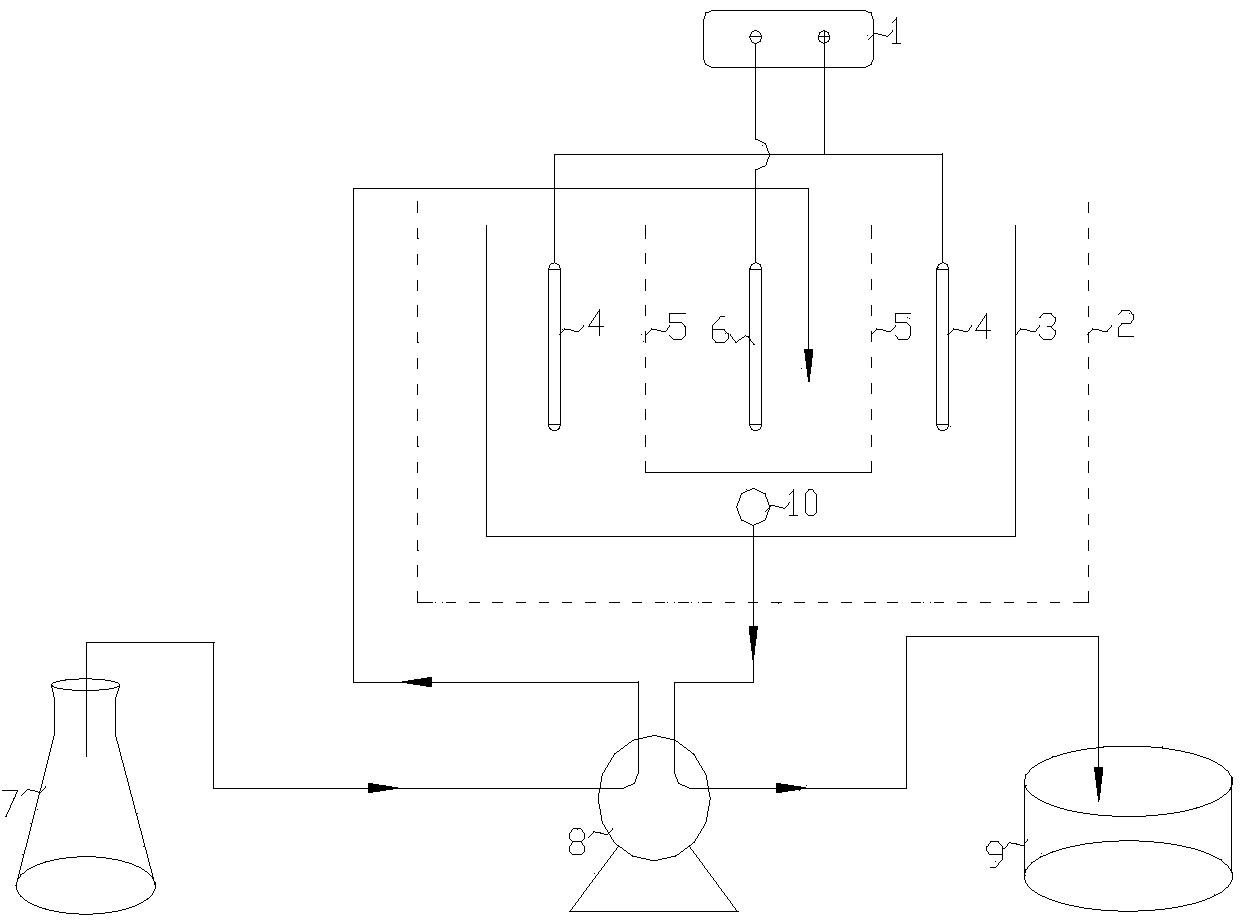
[0034] Put the pyrolusite and pyrite into the ball mill for ball milling respectively, and sieve to obtain the ore powder with a particle size of 150 mesh; mix the pyrolusite powder and pyrite powder with a molar ratio of 1:2, and load them into 4 Among the reaction tubes with gas, all the reaction tubes were placed in the furnace for flame insulation and constant temperature calcination, the calcination temperature was 500°C, and the calcination time was 3 hours. Import the roasted clinker into the pool, and add clear water to leaching the ore at a mass ratio of 1:5. After leaching for 1.5 hours, pass 7 / 8 of the upper layer liquid into the liquid storage tank, pour the lower layer liquid into the leaching tank, and add sulfuric acid according to the mass ratio of 1:0.05, and keep stirring. After 1.5 hours, the secondary leachate was introduced into the liquid storage tank and mixed with the primary leachate, and the leachate in the liquid storage tank was analyzed for all ele...
Embodiment 2
[0037] Put the pyrolusite and pyrite into the ball mill for ball milling, respectively sieve to obtain the ore powder with a particle size of 200 mesh; mix the pyrolusite powder and the pyrite powder with a molar ratio of 1:3, and load them into 4 Among the reaction tubes with gas, all the reaction tubes were placed in the furnace to carry out constant-temperature calcination with a flame insulation, the calcination temperature was 600°C, and the calcination time was 3.5 hours. Import the roasted clinker into the pool, and add clear water to leaching the ore at a mass ratio of 1:5.5. After leaching for 2 hours, pass 9 / 10 of the upper layer liquid into the liquid storage tank, pour the lower layer liquid into the leaching tank, and add sulfuric acid according to the mass ratio of 1:0.06, and keep stirring. After 1 hour, the secondary leachate was introduced into the liquid storage tank and mixed with the primary leachate, and the leachate in the liquid storage tank was analyzed...
Embodiment 3
[0039] Put the pyrolusite and pyrite into the ball mill for ball milling respectively, and sieve to obtain the ore powder with a particle size of 250 mesh; mix the pyrolusite powder and pyrite powder with a molar ratio of 1:4, and load them into 4 Among the reaction tubes with gas, all the reaction tubes were placed in the furnace for flame insulation and constant temperature calcination, the calcination temperature was 700°C, and the calcination time was 4 hours. Import the roasted clinker into the pool, and add clear water to leaching the ore at a mass ratio of 1:6. After leaching for 2.5 hours, pass 9 / 10 of the upper layer liquid into the liquid storage tank, pour the lower layer liquid into the leaching tank, and add sulfuric acid according to the mass ratio of 1:0.07, and keep stirring. After 1 hour, the secondary leachate was introduced into the liquid storage tank and mixed with the primary leachate, and the leachate in the liquid storage tank was analyzed for all eleme...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com