Chemiluminiscence imaging immunoassay method for simultaneously measuring oxidized lipoprotein (a) and oxidized low-density lipoprotein of human serum
A low-density lipoprotein and chemiluminescence technology, used in biological testing, material testing products, etc., can solve the problems of single component detection, difficult clinical promotion, expensive kits, etc. The effect of low sample consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
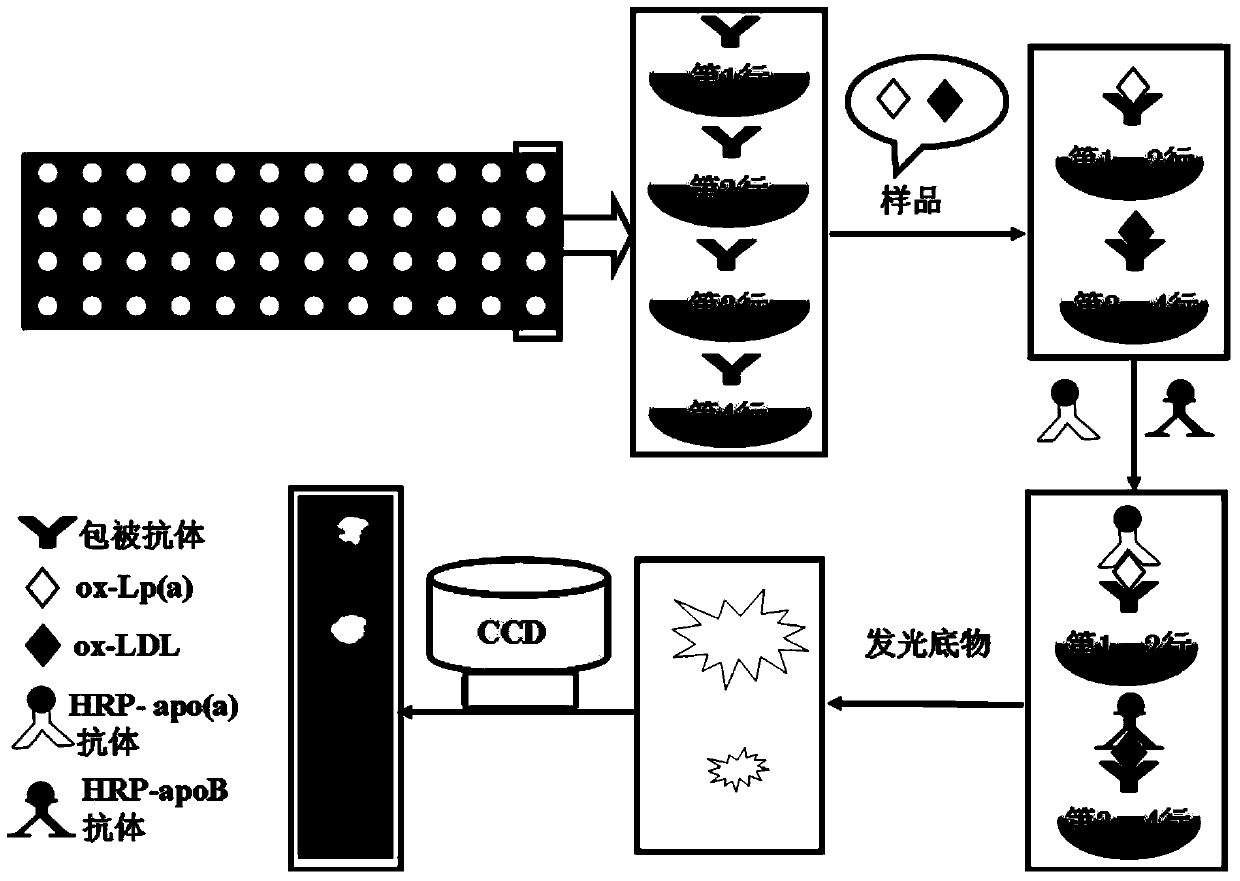
[0036] Example 1: Preparation of Immunosensing Array
[0037] First, piranha acid solution (H 2 SO 4 / 30%H 2 o 2 , the volume ratio is 7:3) to soak the glass slide, and let it stand at room temperature for 12 hours to make the glass slide hydroxylated; then rinse it with ultrapure water, dry it with nitrogen, and immerse the activated slide in 1% (v / v) GPTMS / toluene solution (that is, the volume percentage of the GPTMS solution in the toluene solution is 1%), at room temperature (25°C) overnight to make it silanized. Then, rinse with toluene and absolute ethanol in order to remove silane physically adsorbed on the surface of the slide, and blow dry with nitrogen. Finally, use screen printing to process the glass slide to form 48 microwells (diameter 2mm, hole spacing 4mm, 4 rows x 12 columns) on the surface (see figure 1). The rabbit anti-human ox-LDL antibody was diluted to 0.33μg / mL with pH9.6, 0.05M carbonate coating solution, and added to the microwells of 48-well s...
Embodiment 2
[0038] Embodiment 2: the preparation of standard series solution
[0039] Lipoprotein separation: Density gradient ultracentrifugation separated 100 cases of normal people mixed fresh plasma LDL (d=1.019-1.063g / mL). Lp(a) was separated by ultracentrifugation to separate 1.055-1.110g / mL human plasma lipoprotein components, and then subjected to SePharose6B column chromatography.
[0040] Oxidation of lipoproteins: lipoproteins were fully dialyzed by pH 7.4, 0.01mol / L PBS, adjusted protein concentration to 0.5mg / mL, added CuSO 4 To a final concentration of 40 μmol / L, after incubation at 37°C for 20 h, use 1 mmol / L EDTA-Na 2 , pH7.4, fully dialyzed with 0.01mol / L PBS. The Lowry method was used to determine the protein content of purified ox-Lp(a) and ox-LDL.
[0041] Fresh sera from 50 cases of healthy people were collected and mixed, and the mixed sera were calibrated by ELISA method with known concentrations of purified ox-Lp(a) and ox-LDL. It is stipulated that 1U=1mg oxid...
Embodiment 3
[0043] Example 3: Optimization of immune reaction conditions
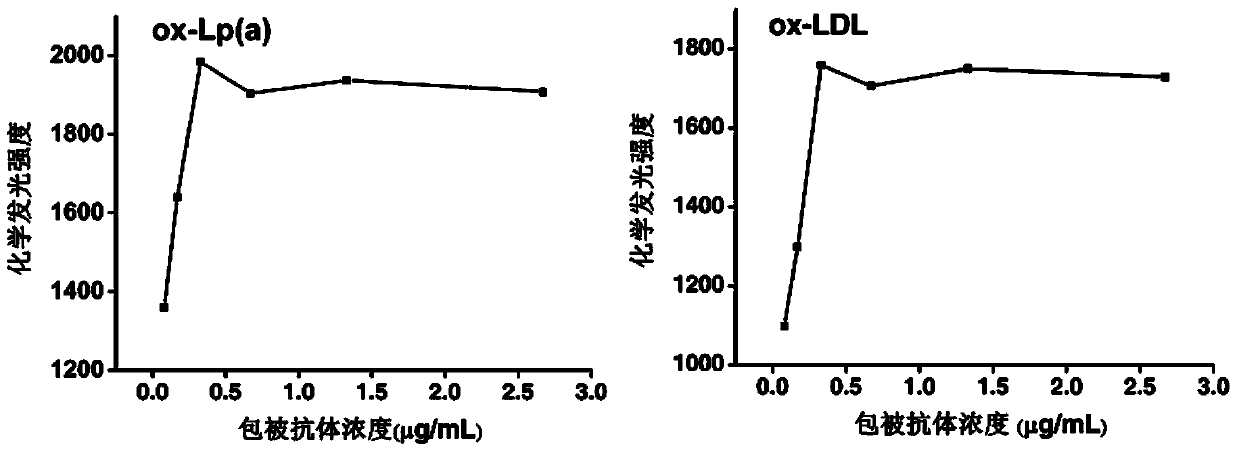
[0044] (1) Effect of coating antibody concentration Dilute the coating antibody concentrated solution at a ratio of 1:375, 1:750, 1:1500, 1:3000, 1:6000 with pH 9.6, 0.05M carbonate buffer 1.5 μL of antibody solutions of different concentrations were added to the microwells of the array, and after overnight at 4° C., they were blocked at room temperature for 1 hour. Preferably, the concentration of the coating antibody is 0.33 μg / mL (see figure 2 ).
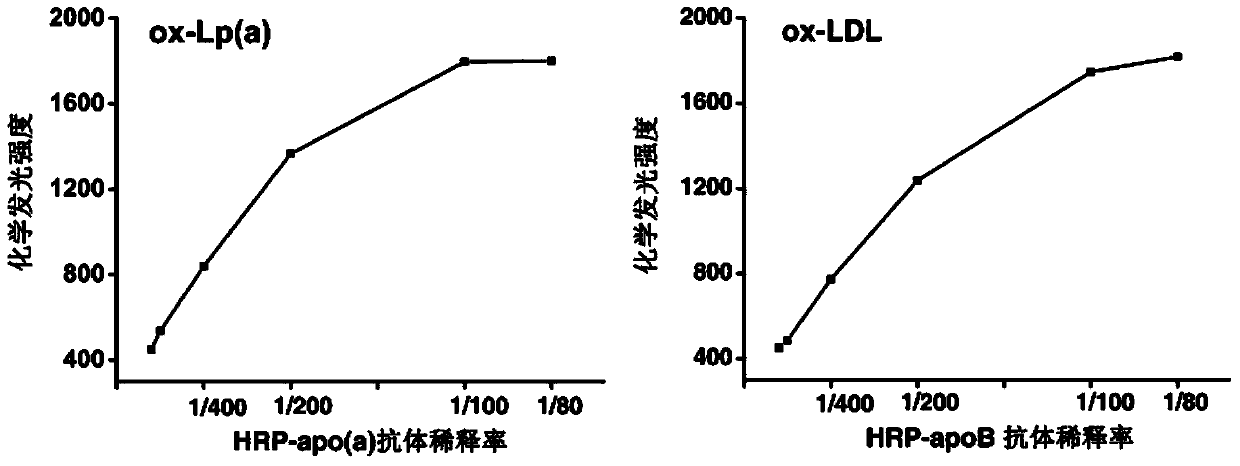
[0045] (2) The influence of the dilution concentration of the enzyme conjugate Dilute the concentrated solution of the enzyme conjugate at the ratio of 1:80, 1:100, 1:200, 1:400, 1:800, 1:1000 (the dilution is containing 5% fetal bovine serum in PBS). As a preference, the dilution ratio of horseradish peroxidase-labeled apolipoprotein (a) [HRP-apo (a)] monoclonal antibody and HRP-apoB polyclonal antibody is 1:100 (see image 3 ).
[0046] (3) Influence of immune...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| Dilution degree | aaaaa | aaaaa |
| Dilution degree | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com