Preparation method and product of azlocillin sodium powder injection
The technology of azlocillin sodium powder and azlocillin sodium is applied in the direction of powder delivery, making medicines into special physical or taking forms, antibacterial medicines, etc. Poor preparation stability and other issues, to achieve the effect of good sample quality, good quality and good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
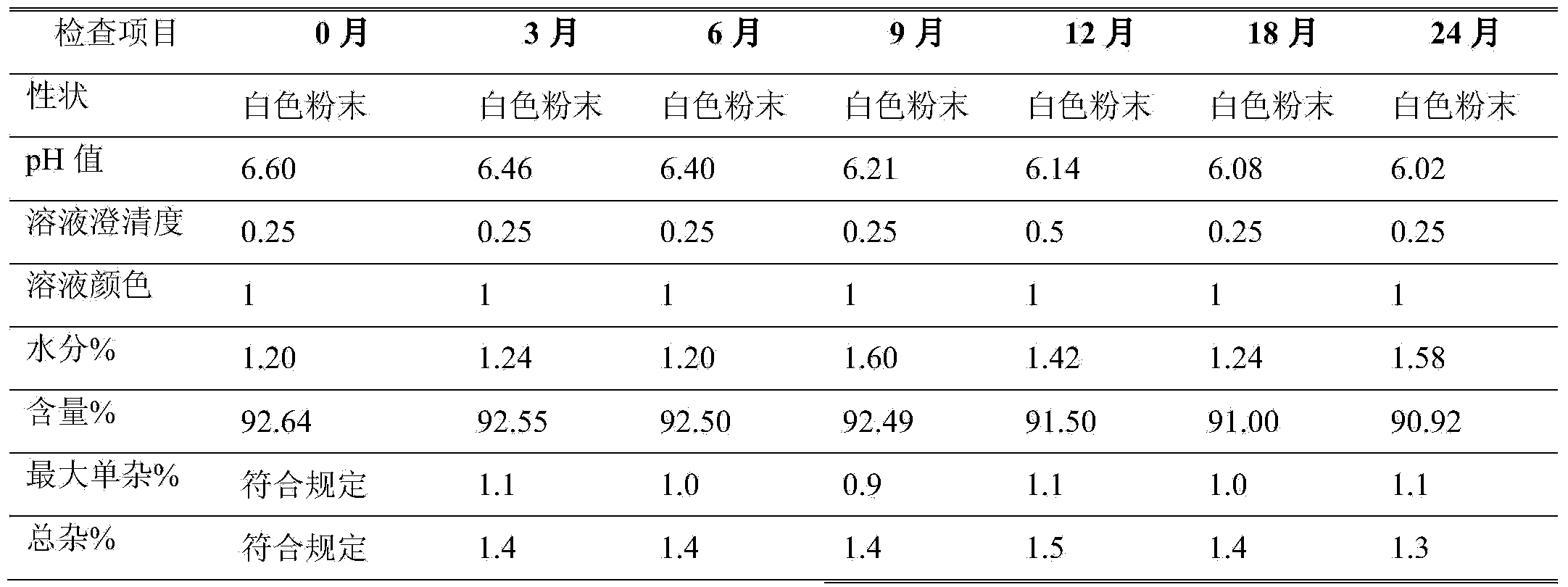
[0012] Example 1 Preparation of Azlocillin Sodium for Injection (1.0g)
[0013] The vials are washed and sterilized by dry heat at 350°C in a tunnel sterilizer before being sent to the packing room. 50.0kg of azlocillin is sieved with a particle size distribution of 60 mesh and 70% to 100% is intercepted. After cleaning and unpacking, the clarity is sent to the In the B-level packaging room, the original drug is packaged into 50,000 sterilized vials with an airflow packaging machine under A-level laminar flow, and the temperature and humidity of the controlled environment are 18-26°C, 45%RH.
Embodiment 2
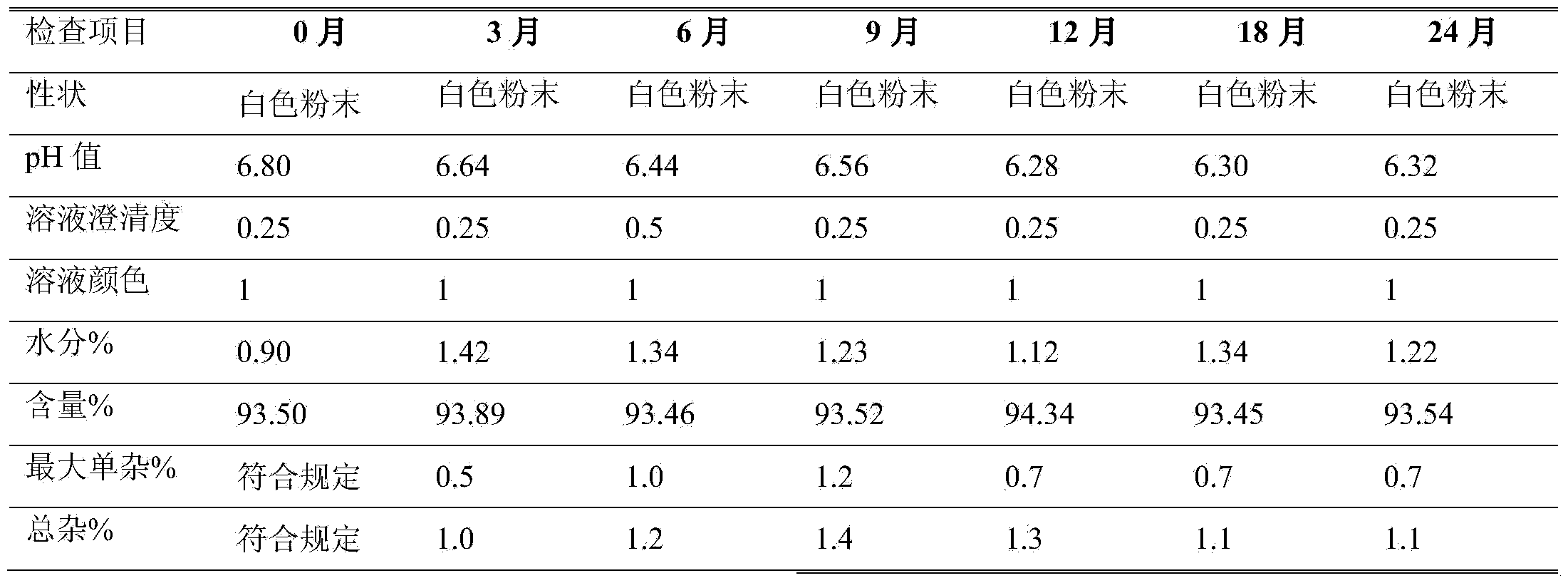
[0014] Preparation of Example 2 Azlocillin Sodium for Injection 2.0g
[0015] The vials are washed and sterilized by dry heat at 350°C in a tunnel sterilizer, and then sent to the packaging room. Take 50.0kg of azlocillin, sieve with a particle size distribution of 60 mesh, cut off 90% to 100% of the cleanliness, and send it to the In the B-level packaging room, the original drug is packaged into 25,000 sterilized vials under the A-level laminar flow using an airflow packaging machine, and the temperature and humidity of the controlled environment are 18-26°C, 45%RH.
Embodiment 3
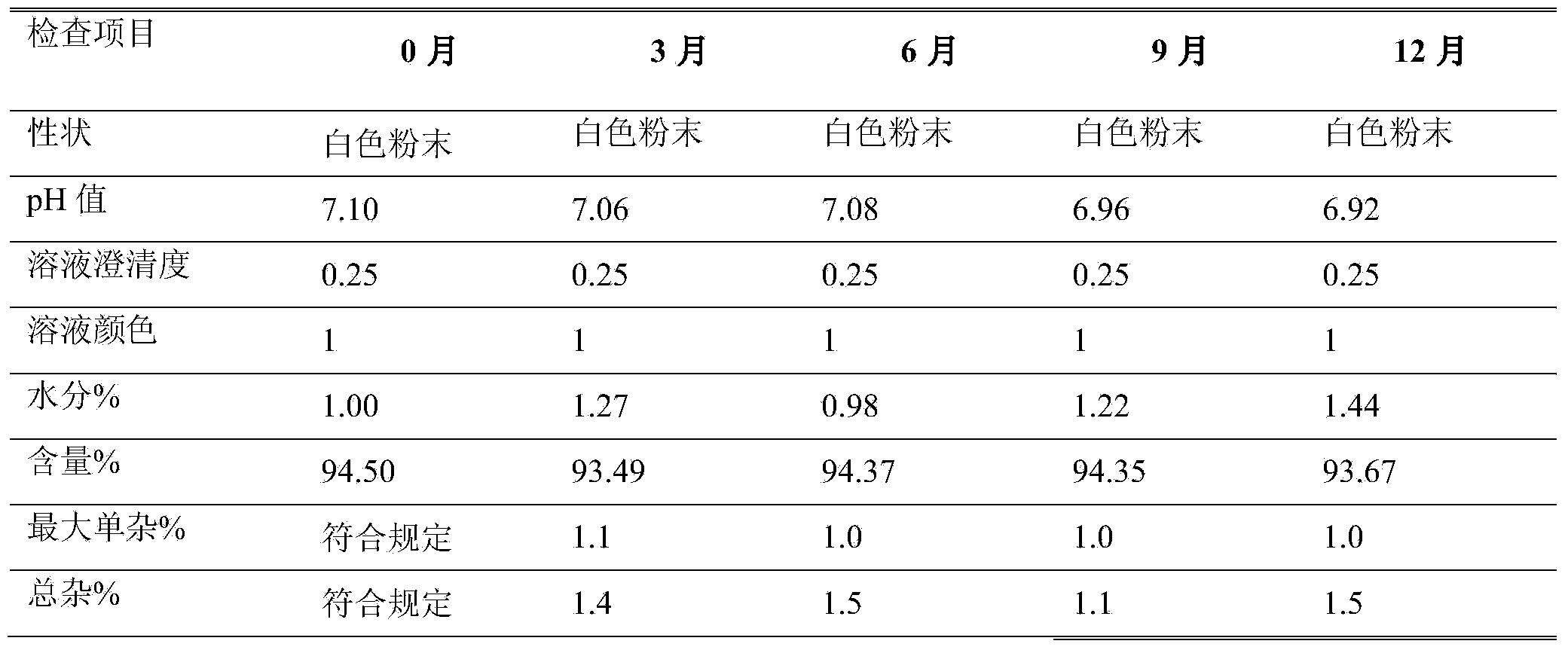
[0016] Preparation of Example 3 Azlocillin Sodium for Injection 2.0g
[0017] The vials are washed and sterilized by dry heat at 350°C in a tunnel sterilizer, and then sent to the packing room. Take 50.0kg of azlocillin, sieve with a particle size distribution of 60 mesh, cut off 70% to 90% of the cleanliness and send it to the In the B-level packaging room, the original drug is packaged in 25,000 sterilized vials with an airflow packaging machine under the A-level laminar flow, and the temperature and humidity of the controlled environment are 25°C and 45%RH.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com