A kind of genetically engineered bacteria highly expressing Aspergillus oryzae prolyl endopeptidase and its application
A high-efficiency expression and gene technology, applied in the field of bioengineering, to achieve good tolerance and increase yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1A
[0041] The acquisition of embodiment 1A.oryzae proline-specific endoprotease gene
[0042] Based on Aeromonas (Genbank: AF065429), Pseudomonas capsular (Genbank: AB010298), Myxococcus flavum (Genbank: ABF89794) and Aspergillus fumigatus (Genbank: XM744168) and Aspergillus niger (Genbank: AX458699) sources The proline-specific endoprotease protein sequence design degenerate primers are as follows:
[0043] g1:5-RASMTWSRYATYASGGRA-3;
[0044] g2: 5-SVBYCBCYMBRGRKMANRNTB-3;
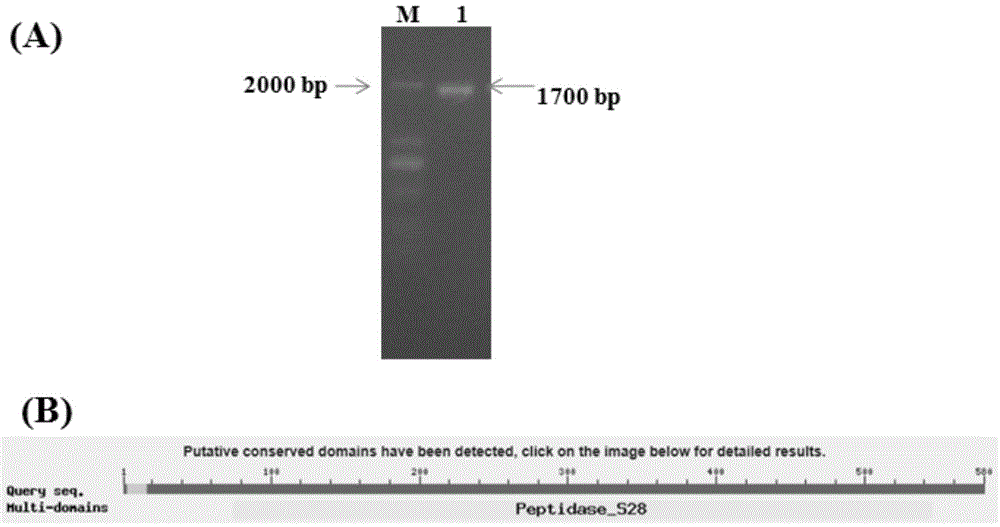
[0045] Using Aspergillus oryzae cDNA as a template and g1 and g2 as primers for PCR, a 1000bp product fragment was obtained. After the product was sequenced and compared with NCBI, it was found that it was all related to the A. oryzae protease gene (Sequence ID: XM_001825944.2 ) with high conserved sequence homology.
[0046] Furthermore, we redesigned primers and performed PCR based on the sequence of the A. oryzae protease gene (Sequence ID: XM_001825944.2) published in the GenBank database. The steps a...
Embodiment 2
[0066] Example 2 Obtaining the Predicted Crystal Structure of A.oryzae Proline-Specific Endoprotease and Its Amino Acid Similarity with Other Aspergillus Proline-Specific Endoproteases Using "Homologous Modeling" Method
[0067] The total length of the Aspergillus oryzae proline-specific endoprotease gene S2 is 1743bp, and the predicted open reading frame of the new protein is located at nucleotides 64-1743, encoding 558 amino acid residues and a molecular weight of 65kDa.
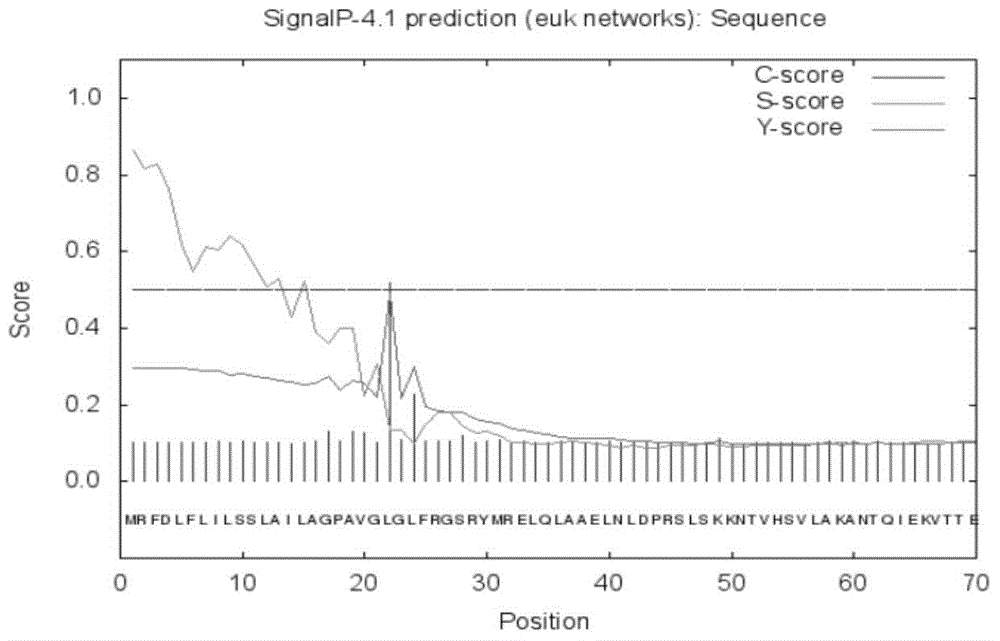
[0068] According to SignalP prediction, the possibility of the N-terminus of the protein being a signal peptide is 86.4%, and the cleavage site of the signal peptide is located between amino acids 21 and 22 (see figure 2 ).
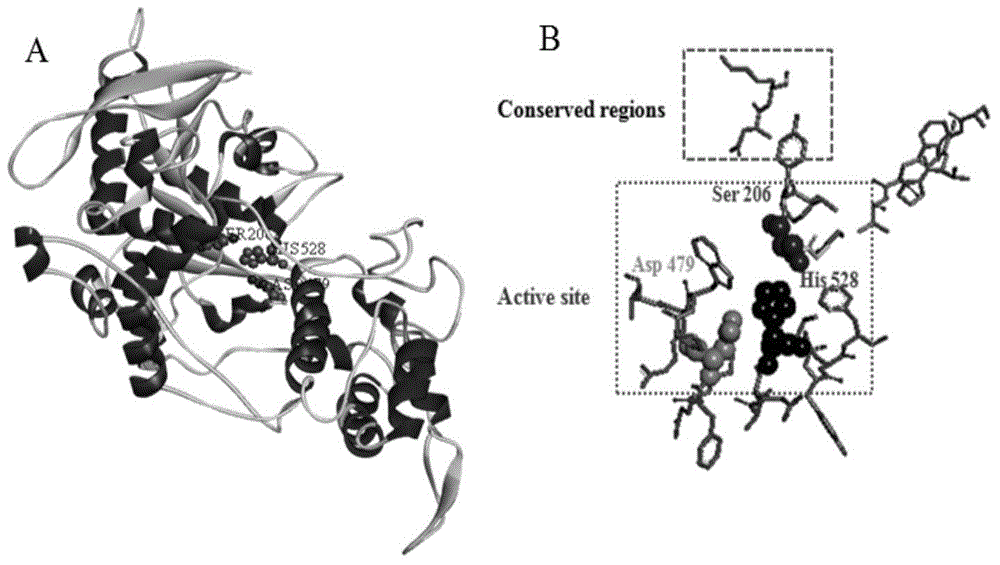
[0069] Submit the amino acid sequence of A. oryzae proline-specific endoprotease to the SWISS-MODEL protein online modeling server (http: / / swissmodel.expasy.org / ) for homology modeling, and then use Discovery studio software to analyze Aspergillus oryzae Proline-specific endoproteas...
Embodiment 3
[0071] Example 3 Construction of Aspergillus oryzae proline-specific endoprotease eukaryotic expression vector, recombinant expression and protein expression thereof
[0072] 1. Construction of eukaryotic expression vector
[0073] 1) Primer design: design primers starting from the mature peptide sequence after the signal peptide
[0074] g5:5'-CGG TACGTA TTGGGGT TGTTTAGAGG-3';
[0075] g6:5'-CC GCGGCCGC CTACATCACCGCCCCCTTTG-3';
[0076] 2) PCR reaction, using the cloning vector pMD-19T-S2 as a template, annealing at 55-62°C, 35 cycles.
[0077] 3) SnaBI and NotI double-digest the PCR product of S2 and plasmid pPIC9
[0078] Element
Usage amount
Purification of PCR products / plasmids
30μl
10*quitcut buffer
5μl
QuitCut SnaBI
1μl
Quit Cut Not I
1μl
wxya 2 o
13μl
total capacity
50μl
[0079] Digest at 37℃ for 2hr
[0080] 4) Ligate, transform, and identify by double enzyme digestion ac...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com