Recombinant porcine circovirus type 2 virus-like particle, and preparation method and application thereof
A porcine circovirus and type 2 virus technology, applied in the fields of botanical equipment and methods, biochemical equipment and methods, applications, etc., can solve the problems of short duration of immunity, unsatisfactory immune effect, and poor immune effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
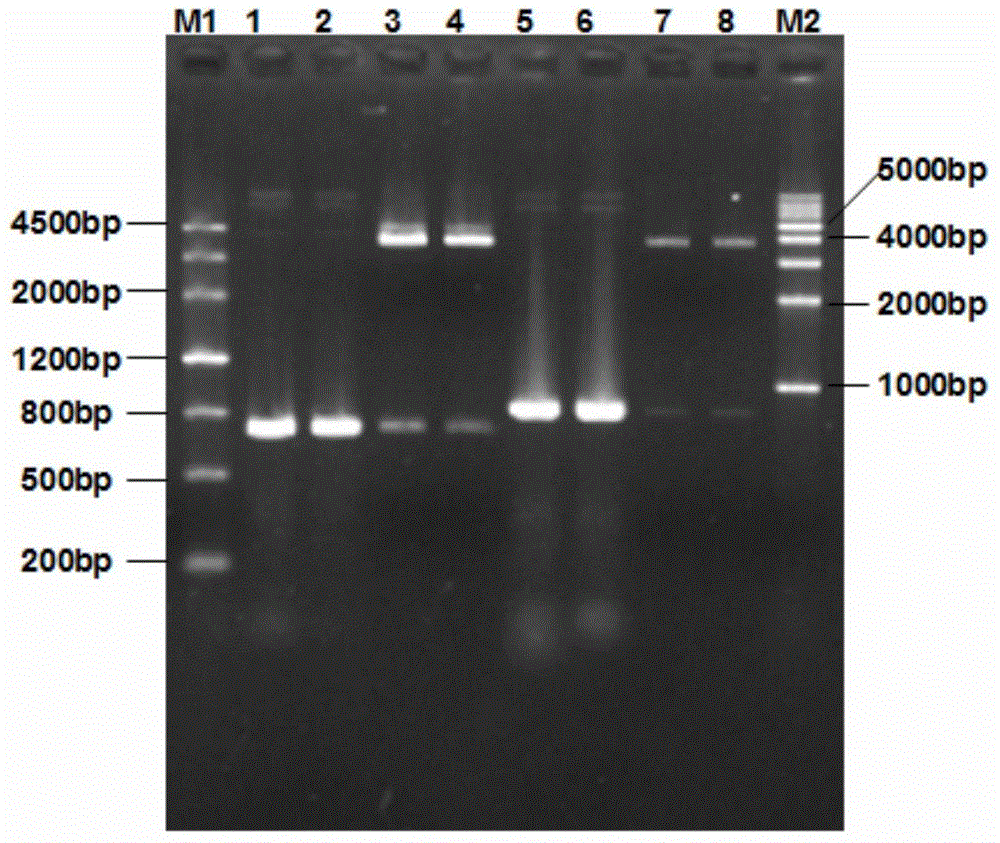
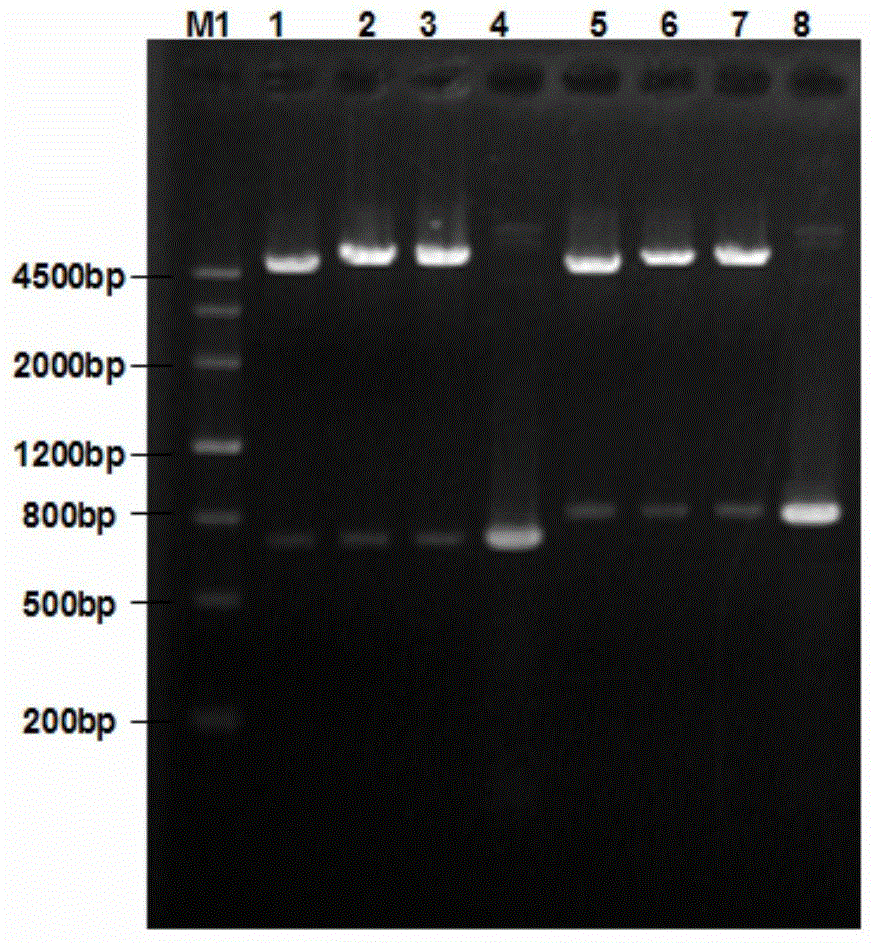
[0030] The recombinant porcine circovirus type 2 virus-like particle and its subunit vaccine of the present invention are constructed as follows:
[0031] 1. Research Methods
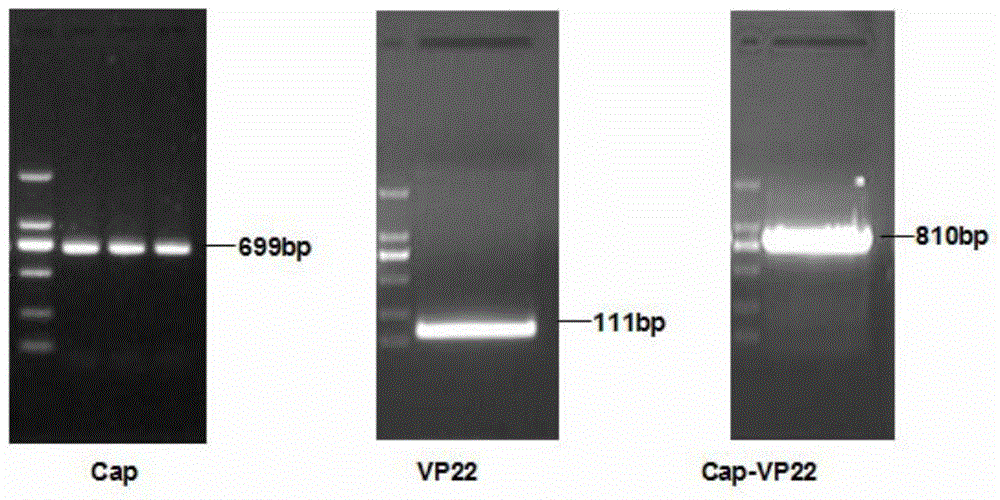
[0032] 1. Amplification and cloning of the Cap-VP22 gene. According to the PCV2 (GenBank accession number: GU450328) ORF2 sequence published by GenBank, specific primers for amplifying the Cap protein gene were designed and synthesized, and the VP22 protein transduction domain sequence was synthesized, and the Cap Primer for fusion of protein gene sequence and VP22 protein transduction domain sequence. The PCV2 virus DNA was extracted by conventional methods, and the Cap-VP22 fusion gene was amplified by SOE-PCR method. After the fusion gene was sequenced, the two Cap-VP22 genes were inserted into the rod successively through BamHI / NotI and NheI / KpnI digestion. In the multiple cloning site of the virus transfer vector pFastBac Dual, a positive recombinant baculovirus transfer vector pFastBac Dual-2Cap-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com