Thymalfasin sustained-release microspheres and preparation method thereof
A thymosin, slow-release microsphere technology, applied in the field of medicine, can solve the problems of reduced product purity, solvent residue, preparation hazards, etc., and achieve the effect of stable drug release, less impurities, and continuous release.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
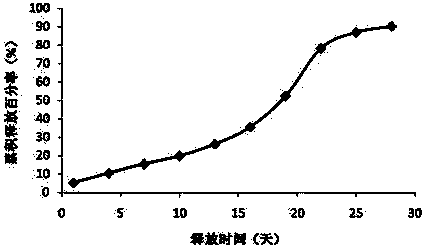
[0035] Example 1: Preparation of new sustained-release microspheres of thymus method of the present invention
[0036] The commercially available Thymus Faxin is dissolved in water, filtered and sterilized, and then lyophilized and sieved to collect Thymus Faxin powder below 500 mesh. PLGA (polylactic acid glycolic acid, LA:GA=50:50, purchased from Lakeshore Biomaterials, intrinsic viscosity 0.15-0.25dL / g) was pulverized by a ball mill at 10℃, and then sieved to collect powder below 80 mesh . The trehalose is pulverized by a ball mill, and then sieved to collect powder below 500 mesh.
[0037] Add 40g of PLGA, 0.2g of trehalose and 3g of thymus method powder into the mixer and mix for 10 minutes. Put the above mixture into a twin-screw extruder (purchased from Thermo Fisher Scientific), and adjust the melt extrusion temperature of the four sections of the extruder at different temperature control zones to 80°C (rotating speed 160 revolutions per minute). Carry out heating compre...
Embodiment 2
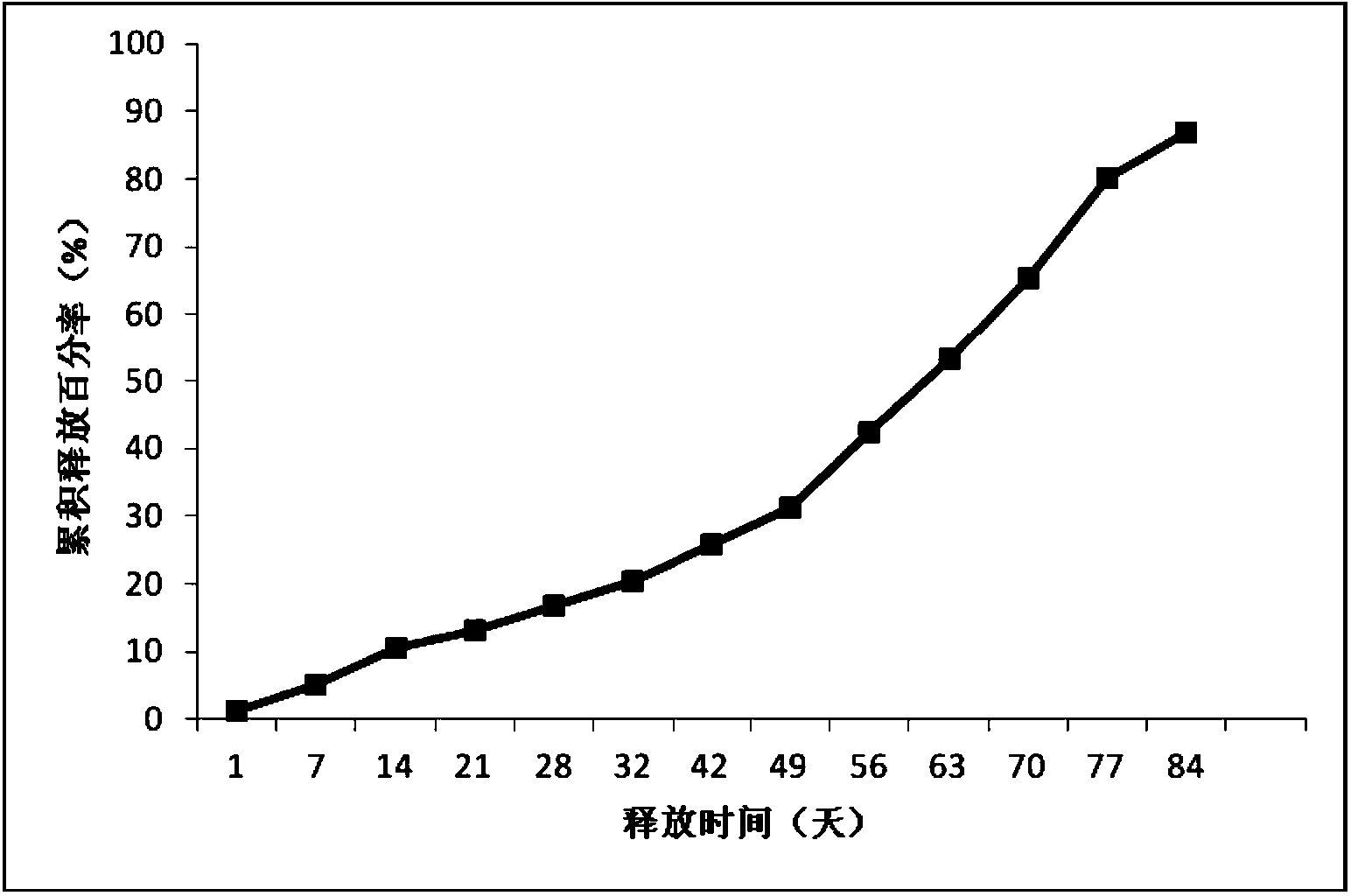
[0039] Example 2: Preparation of new sustained-release microspheres of thymus method of the present invention
[0040] The commercially available Thymus Faxin is dissolved in water, filtered and sterilized, and then lyophilized and sieved to collect Thymus Faxin powder below 500 mesh. The PLGA (polylactic acid glycolic acid, LA: GA=75:25, purchased from Lakeshore Biomaterials, with an intrinsic viscosity of 0.32-0.44dL / g) was pulverized by a ball mill at 12°C, and then sieved to collect powder below 80 mesh .
[0041] Add 40g of PLGA and 2g of Thymus Faxin powder into the mixer and mix for 10 minutes. Put the above mixture into a twin-screw extruder (purchased from Thermo Fisher Scientific), adjust the melt extrusion temperature of the four sections of the extruder at different temperature control zones to 85°C (rotating speed 160 revolutions per minute). Carry out heating compression melt extrusion, the obtained molten extrudate is drawn into a long strip with a diameter of 2mm ...
Embodiment 3
[0043] Example 3: Preparation of new sustained-release microspheres of thymus method of the present invention
[0044] The commercially available Thymus Faxin is dissolved in water, filtered and sterilized, and then lyophilized and sieved to collect Thymus Faxin powder below 500 mesh. The polylactic acid (intrinsic viscosity 0.25-0.35dL / g, purchased from Lakeshore Biomaterials) was pulverized by a ball mill at 12° C., and then sieved to collect powder with 80 meshes or less.
[0045] Add 30 g of polylactic acid and 2 g of thymus method powder into the mixer and mix for 10 minutes. Put the above mixture into a twin-screw extruder (purchased from Thermo Fisher Scientific), and adjust the melt extrusion temperature of the four sections of the extruder at different temperature control zones to 95°C (rotating speed 160 revolutions per minute). Carry out heating compression melt extrusion, the obtained molten extrudate is drawn into a long strip with a diameter of 2mm on a tractor, and ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Intrinsic viscosity | aaaaa | aaaaa |
| Intrinsic viscosity | aaaaa | aaaaa |
| Intrinsic viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com