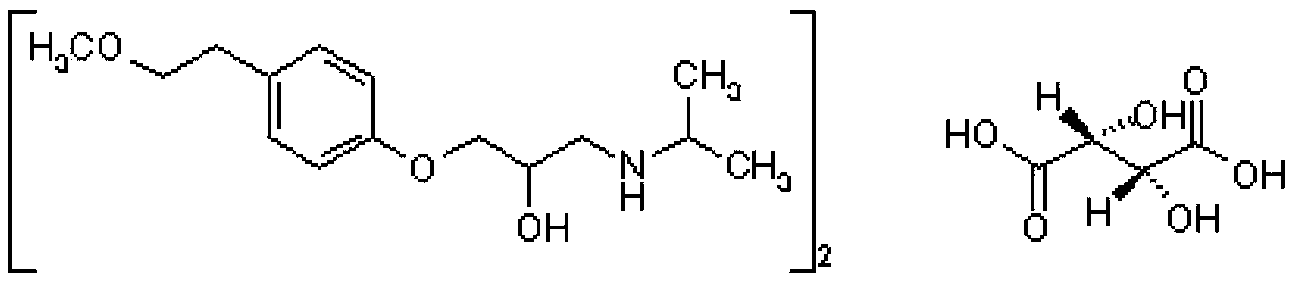
Metoprolol tartrate sustained release tablet and preparation method thereof
A technology for metoprolol tartrate and torolol sustained-release tablets, which can be used in pharmaceutical formulations, medical preparations with inactive ingredients, and medical preparations containing active ingredients, etc., and can solve problems such as sticking and impacting production, etc. , to achieve the effect of strong hygroscopicity, improved stability and good fluidity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Preparation: Put metoprolol tartrate and hypromellose E50 in a KJZ-10 fast stirring granulator, stir rapidly for 5 minutes to mix evenly, add 220g of 80% ethanol solution and stir rapidly for 5 minutes to make a soft material; then YK -160 oscillating granulator 20 mesh sieve granulation, as drug-containing granules; mix lactose and hypromellose K4M evenly, 80% ethanol solution 440g, the same method as the soft material, granulate, as blank granules; drug-containing granules and Blank granules were dried in a CT-C-F hot air circulation oven at 60±5°C for 7 hours, passed through a YK-160 oscillating granulator with a 20-mesh sieve for granulation, added silicon dioxide and magnesium stearate, mixed evenly, and ZP33 rotary tabletting Machine, Φ8.5mm round concave punching tablet, the film coating powder (stomach-soluble type) purchased from Shanghai Colorcon Coating Technology Co., Ltd. is added to the stirred 30% ethanol solution to make film coating powder (Stoma...
Embodiment 2
[0031] Preparation: Put metoprolol tartrate and hypromellose E50 in a KJZ-10 fast stirring granulator, stir rapidly for 5 minutes to mix evenly, add 230g of 80% ethanol solution, and stir rapidly for 5 minutes to make a soft material; then YK-160 oscillating granulator 20 mesh sieve granules, as drug-containing granules; mix lactose and hypromellose K4M evenly, 80% ethanol solution 430g, the same method as the soft material, granulate, as blank granules; drug-containing granules and blank granules were dried in a CT-C-F hot air circulation oven at 60±5°C for 7 hours, passed through a YK-160 swinging granulator with a 20-mesh sieve for granulation, added silicon dioxide and magnesium stearate and mixed evenly, and ZP33 rotary press Tablet machine, Φ8.5mm round concave punched tablets, film coating powder (stomach-soluble type) purchased from Shanghai Colorcon Coating Technology Co., Ltd., added to the stirred 30% ethanol solution to make film coating powder (Stomach-solu...
Embodiment 3
31g
[0037] Preparation: Put metoprolol tartrate and hypromellose E50 in a KJZ-10 fast stirring granulator, stir quickly for 5 minutes to mix evenly, add 200g of 80% ethanol solution and stir quickly for 5 minutes to make a soft material; YK- Granulate with a 20-mesh sieve in a 160 swinging granulator, and use it as a drug-containing granule; mix lactose and hypromellose K4M evenly, add 460g of 80% ethanol solution, and use the same method as the soft material, granulate, and use it as a blank granule; the drug-containing granule and blank The granules were dried in a CT-C-F hot air circulation oven at 60±5°C for 7 hours, passed through a YK-160 swinging granulator with a 20-mesh sieve for granulation, added silicon dioxide and magnesium stearate and mixed evenly, and ZP33 rotary tablet press , Φ8.5mm round concave punched tablet, film coating powder (gastric dissolving type) purchased from Shanghai Colorcon Coating Technology Co., Ltd., added to the stirred 30% ethan...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com