Proton pump inhibitor enteric coated pellet and preparation and preparation method thereof
A proton pump inhibitor, proton pump technology, applied in the direction of pharmaceutical formulations, non-active ingredients of polymer compounds, medical preparations containing active ingredients, etc. And other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
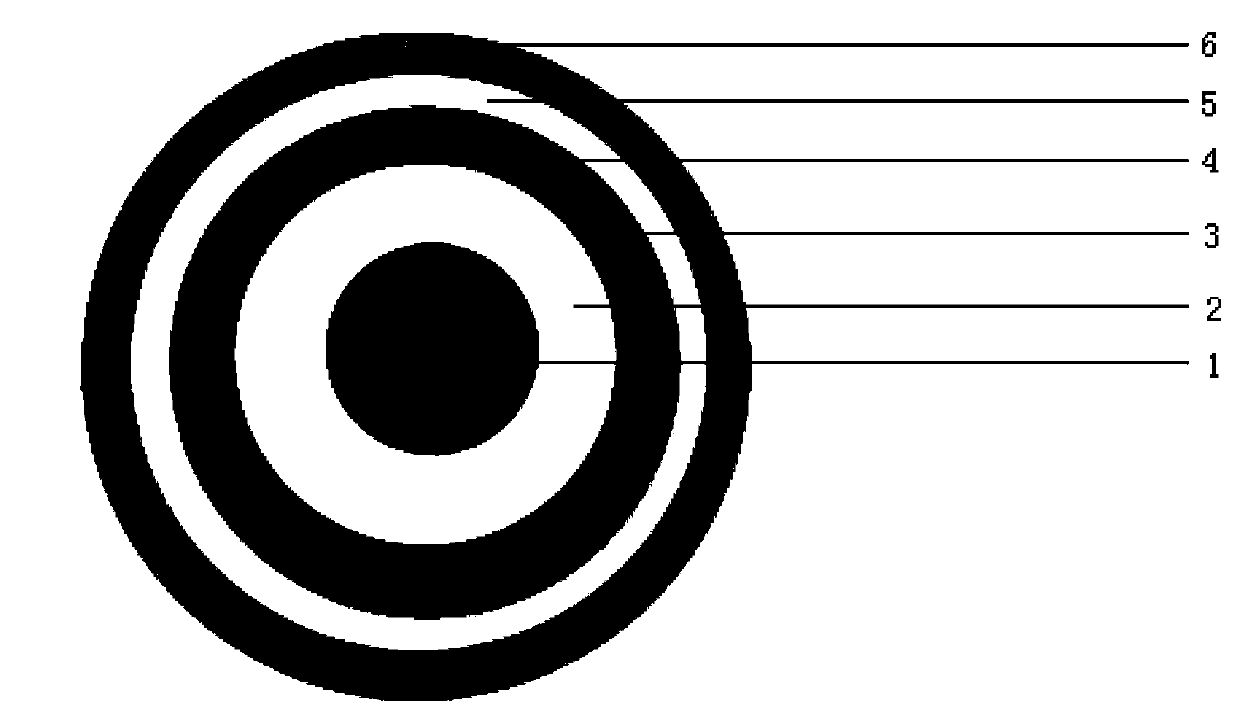
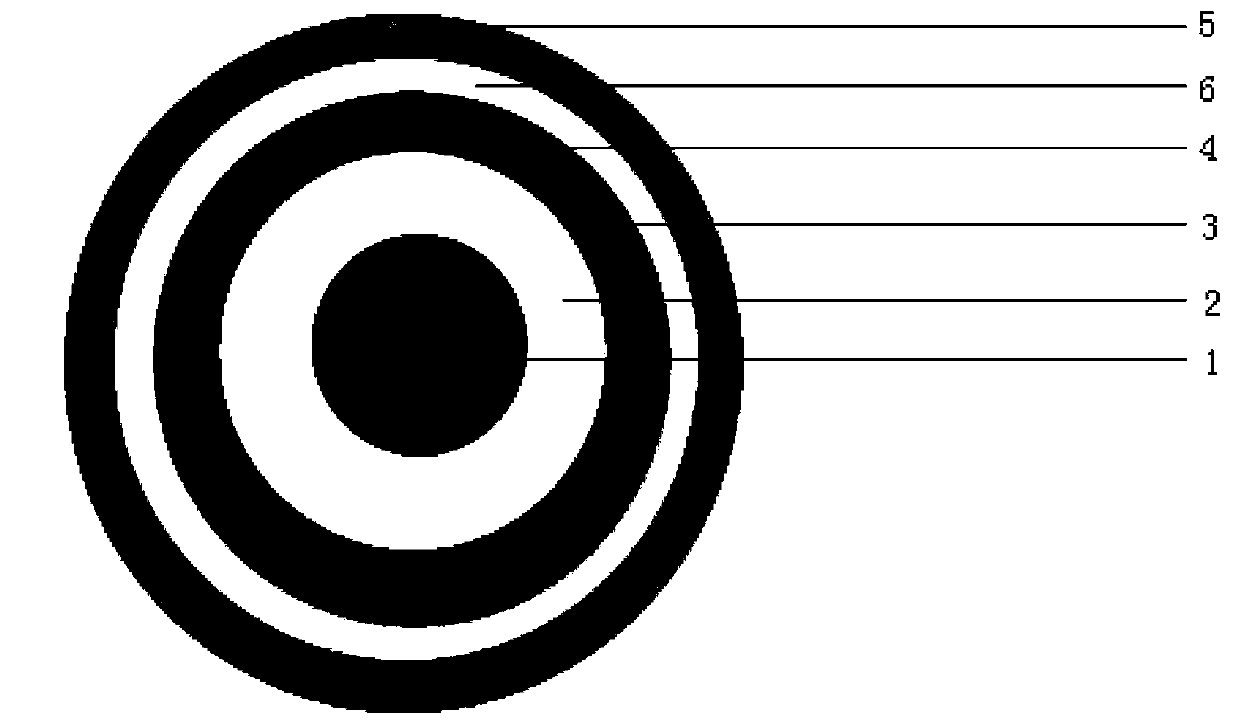
Image
Examples
Embodiment 1
[0144] The preparation of embodiment 1 omeprazole enteric-coated pellets
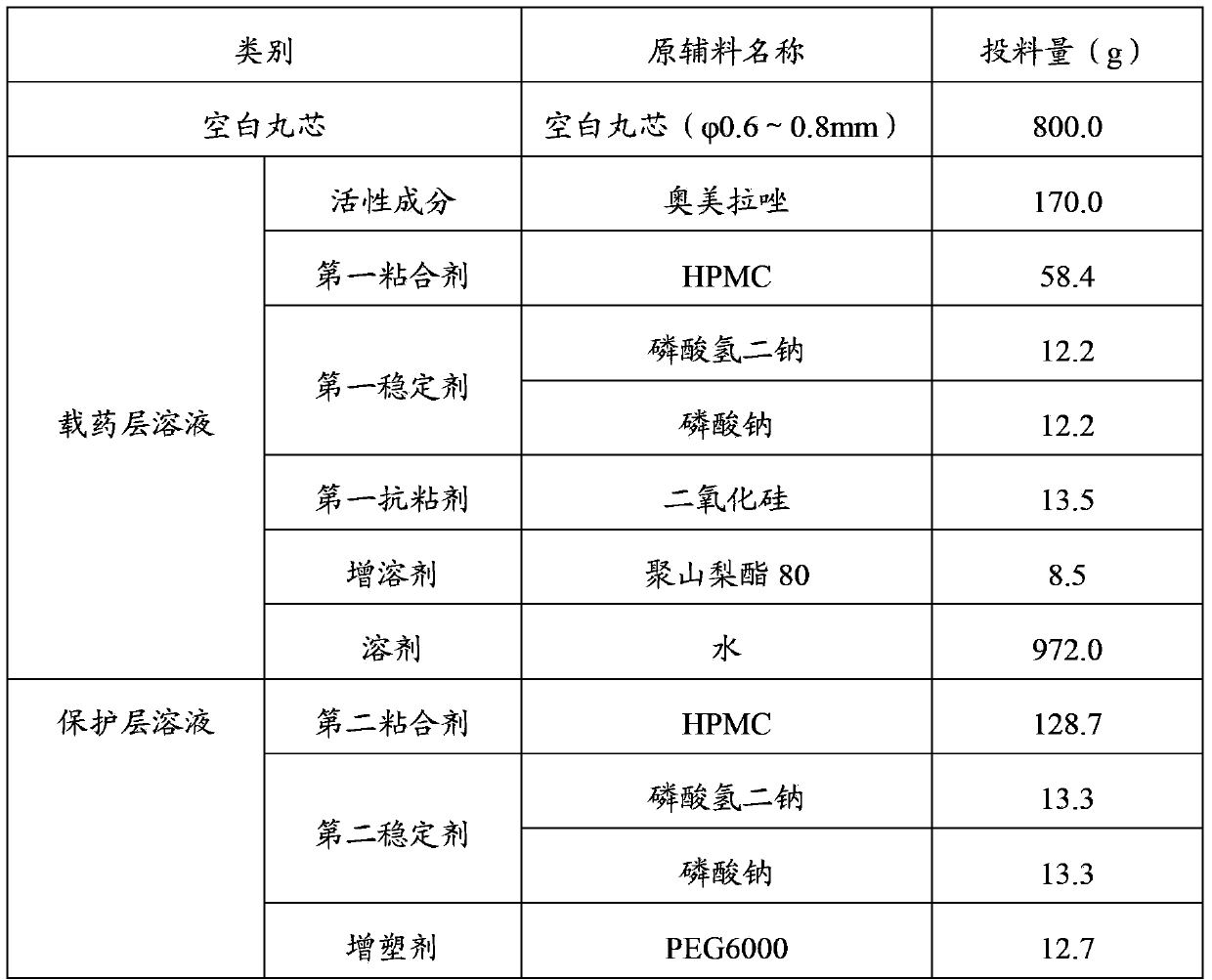
[0145]Add 58.4g of HPMC to 500g of purified water at 25°C, stir and swell until clear and ready for use; weigh 12.2g of disodium hydrogen phosphate and 12.2g of sodium phosphate and dissolve them in 50g of purified water, and add the above-mentioned hydrogen phosphate in turn while stirring the HPMC solution Disodium solution and sodium phosphate solution; 8.5 g of Tween 80 and 13.5 g of silicon dioxide were added while stirring; 170.0 g of omeprazole was added and stirred evenly to obtain a suspension. Pass the above suspension through a 80-mesh sieve, rinse it with 100 g of purified water, add 272.0 g of purified water, and stir evenly to obtain a drug-loaded layer solution. Fill 800g of sucrose-starch microspheres (particle size 0.6~0.8mm) into the fluidized bed granulation coating machine, fan frequency 20~40Hz, air inlet temperature 50~70℃, material temperature 35~50℃, atomization The air pressure...
Embodiment 2
[0154] The preparation of embodiment 2 Lansoprazole enteric-coated pellets
[0155] 300.0g lansoprazole, 105.0g magnesium carbonate, 195.0g purifying sucrose and 75.0g low-substituted hydroxypropyl cellulose are evenly mixed to obtain dusting powder for the active ingredient layer; 75.0g purifying sucrose, 48.8g titanium dioxide and 18.8g of low-substituted hydroxypropyl cellulose is evenly mixed to obtain the dusting powder for the middle layer; 9.4g of hydroxypropyl cellulose is dissolved in 469.0g of purified water to prepare a hydroxypropyl cellulose solution; 375.0g of sucrose-starch Fill the microspheres into a fluidized bed granulator and coater, and while spraying the hydroxypropyl cellulose solution, use the above-mentioned dusting bag for the active ingredient layer and the dusting bag for the middle layer to coat the sucrose-starch microspheres coating, the obtained pellets coated with the drug-loaded layer solution; set the material temperature at 40°C, fluidize an...
Embodiment 3
[0164] The preparation of embodiment 3 esomeprazole magnesium enteric-coated pellets
[0165] Take 61.0g of HPMC and add it into 500.0g of purified water at 25°C, stir and swell until clear and ready for use; weigh 11.5g of disodium hydrogen phosphate and 11.5g of sodium phosphate and dissolve them in 50.0g of purified water, and add the above-mentioned ones in turn while stirring the HPMC solution. Disodium hydrogen phosphate solution and sodium phosphate solution; add 8.0 g of Tween 80 and 14.0 g of silicon dioxide while stirring; add 162.0 g of esomeprazole magnesium (equivalent to 145.7 g of esomeprazole), Stir well to obtain a suspension. Pass the above suspension through an 80-mesh sieve, rinse it with 100.0 g of purified water, add 223.0 g of purified water, and stir evenly to obtain a drug-loaded layer solution. Fill 700.0g of sucrose-starch microspheres (particle size 0.6~0.8mm) into the fluidized bed granulation coating machine, fan frequency 20~40Hz, air inlet temp...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle size (mesh) | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com