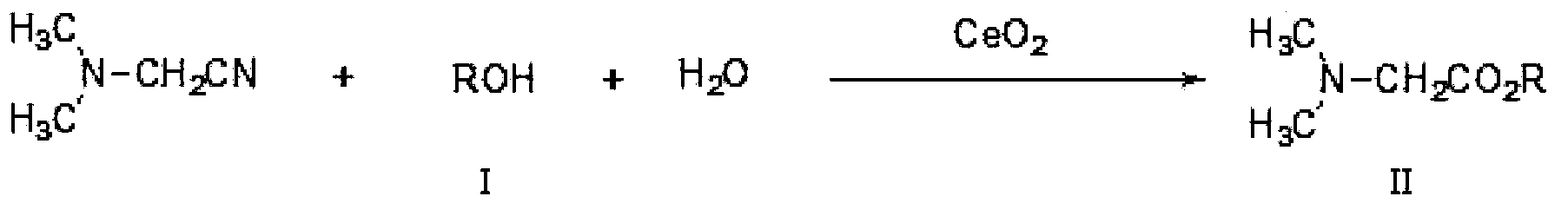One-pot method of preparing N,N-dimethyl glycinate
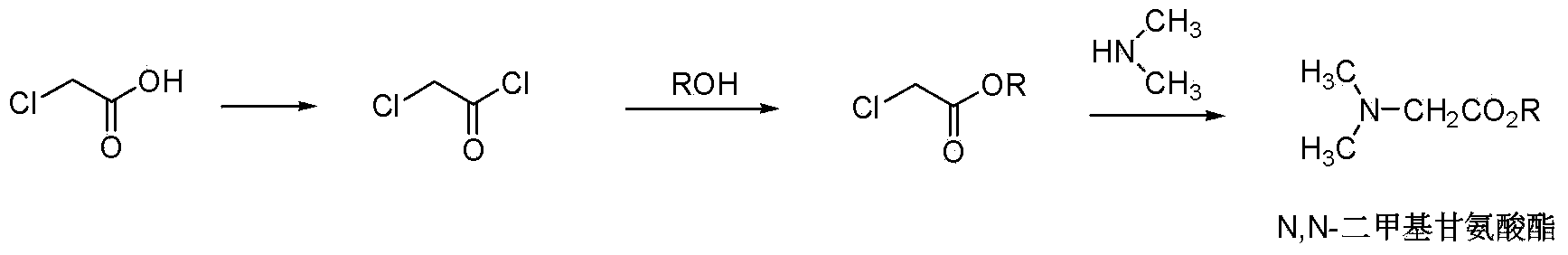
A technology of dimethyl glycine ester and dimethyl amino acetonitrile, which is applied to the chemical industry, can solve the problems of long reaction time, unsafe operation and high reaction cost, and achieves a reaction with less contact equipment, high purity and simple operation steps. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Embodiment 1N, the preparation of N-dimethylaminoacetonitrile
[0038] At room temperature, add 1000 grams of 40% hydroxyacetonitrile aqueous solution to the autoclave, pass through 1435 grams of 33% dimethylamine aqueous solution, then raise the temperature to 60 ° C, pressurize to 0.7 MPa, react for 30 minutes, stop the reaction, and obtain Aqueous solution of N,N-dimethylaminoacetonitrile. The obtained N,N-dimethylaminoacetonitrile aqueous solution was purified to obtain N,N-dimethylaminoacetonitrile with a content of 98%.
Embodiment 2
[0039] Embodiment 2N, the preparation of N-dimethylglycine ethyl ester
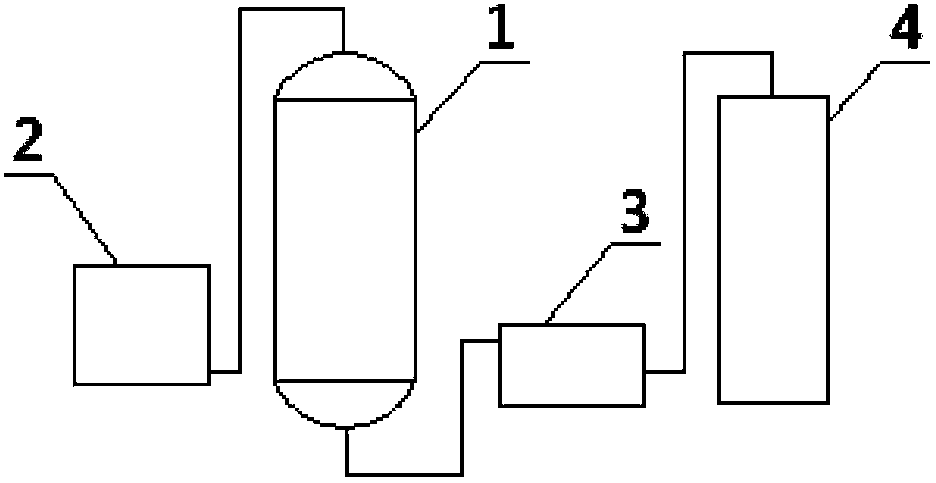
[0040] Add 86 grams of N,N-dimethylaminoacetonitrile, 460 grams of ethanol, 18 grams of water, and 17 grams of catalyst cerium oxide into the reactor, install molecular sieves on the top of the reactor, stir and heat to 140°C under nitrogen protection. The whole reaction device is in an open state, which is convenient for the by-product ammonia gas to be released in time, and the ammonia gas is absorbed with water. The reaction process was detected by gas chromatography until the residual amount of N,N-dimethylaminoacetonitrile was less than 1%. After reacting for 24 hours, stop the reaction, cool, and filter to obtain the crude N,N-dimethylglycine ethyl ester and recovered cerium oxide. Evaporate the crude N,N-dimethylglycine ethyl ester under reduced pressure, control the temperature at 60-100°C, and vacuum degree 0.05MPa, first evaporate light components (mainly including ethanol and residual N,N-dime...
Embodiment 3
[0041] Embodiment 3N, the preparation of N-dimethylglycine isopropyl ester
[0042]Add 86 grams of N,N-dimethylaminoacetonitrile, 600 grams of isopropanol, 18 grams of water, and 20 grams of catalyst cerium oxide into the reactor, install molecular sieves on the top of the reactor, stir and heat to 150°C under nitrogen protection. The whole reaction device is in an open state, which is convenient for the by-product ammonia gas to be released in time, and the ammonia gas is absorbed with water. The reaction process was detected by gas chromatography until the residual amount of N,N-dimethylaminoacetonitrile was less than 1%. After reacting for 24 hours, stop the reaction, cool, and filter to obtain the crude product of N,N-dimethylglycine isopropyl ester and the first recovered cerium oxide. Evaporate the crude N,N-dimethylglycine isopropyl ester, first distill off the light components (mainly including isopropanol and residual N,N-dimethylaminoacetonitrile), then increase the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com