Thymopent aqueous solution preparation and use thereof
A technology of aqueous solution and thymus, which is applied in the application of medicine, pharmaceutical preparations, and the field of aqueous solution preparations of thymopentin, can solve the problems of unable to maintain the stability of the solution, shorten the time of stable storage, and lack of drugs in patients, so as to prevent free calcium The effect of the rapid decrease of the concentration, the reduction of the cost of the drug, and the timely demand for the drug
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
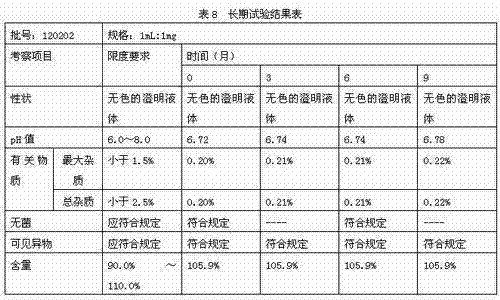
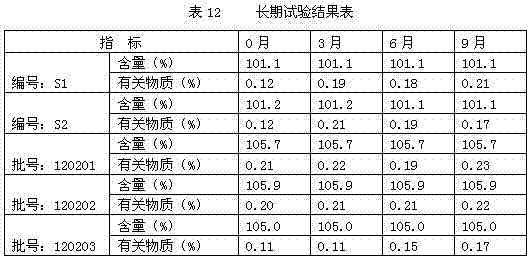
[0038] Accurately weigh 0.1g of thymopentin, 0.015g of sodium acetate, 1.485g of glacial acetic acid, 0.5g of calcium sodium edetate, and 0.3g of sodium chloride, dissolve them in water for injection, and finally dissolve them to 1000ml. The ampoules are divided into 1000 sticks. The final specification is 1ml / branch.
Embodiment 2
[0040] Accurately weigh 100g of thymopentin, 9.655g of sodium acetate, 990.345g of glacial acetic acid, 40g of calcium sodium ethylenediaminetetraacetate, and 200g of sodium chloride, dissolve them in water for injection, and finally dissolve them to 1000ml, and divide them into 2ml glass ampoules. Packed into 1000 pieces. The final specification is 1ml / branch.
Embodiment 3
[0042] Accurately weigh 10g of thymopentin, 0.966g of sodium acetate, 99.034g of glacial acetic acid, 10g of calcium sodium edetate, and 20g of sodium chloride, dissolve them in water for injection, and finally dissolve them to 1000ml. Packed into 1000 pieces. The final specification is 1ml / branch.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com