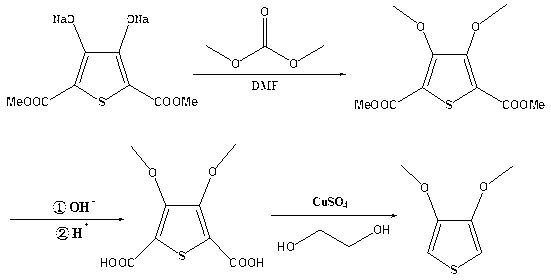
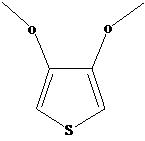
Process for synthesizing 3, 4-dimethoxythiophene
A technology of dimethoxythiophene and a synthesis method, applied in the direction of organic chemistry and the like, can solve problems such as affecting the quality and use of EMOT products, high residual bromide ion content, unfavorable industrial production, etc., achieving convenient industrial production and safe and reliable synthesis process. , the effect of easy industrial production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Add 552.3 g (2.0 mol) of sodium 2,5-dicarboxylate-3,4-thiophenediol to 1600 g of N,N-dimethylformamide, stir at room temperature, and dropwise add 216.2 g of dimethyl carbonate ( 2.4mol), heat up to reflux, and react for 4 hours; after the reaction is completed, evaporate the solvent and add 2000g (5.0mol) of sodium hydroxide solution with a mass concentration of 10% to mix evenly, heat to 85°C, react for 2 hours, and the reaction is complete Finally, acidify with sulfuric acid with a mass concentration of 50% at room temperature until the pH is 1 to 2, filter, add 1200 g of ethylene glycol solvent to the filter residue, add 6.4 g (0.04 mol) of copper sulfate under stirring, and heat to 100 ° C , heat preservation reaction for 3 hours, after the reaction was completed, 211.8g of the product, namely 3,4-dimethoxythiophene, was obtained by rectification under reduced pressure, with a content of 99.0% and a total yield of 72.7%. The ethylene glycol can be reused after recov...
Embodiment 2
[0030] Add 552.3 g (2.0 mol) of sodium 2,5-dicarboxylate-3,4-thiophenediol to 1100 g of N,N-dimethylformamide, stir at room temperature, and dropwise add 540.4 g of dimethyl carbonate ( 6.0mol), heated to reflux, and reacted for 2 hours; after the reaction was completed, evaporate the solvent and add 2400g (6.0mol) of sodium hydroxide solution with a mass concentration of 10%, mix evenly, heat to 90°C, react for 2 hours, and the reaction was completed Finally, acidify with sulfuric acid with a mass concentration of 50% at room temperature to a pH of 1 to 2, filter, add 1600 g of ethylene glycol solvent to the filter residue, add 16.0 g (0.1 mol) of copper sulfate under stirring, and heat to 120 ° C , heat preservation reaction for 6 hours, after the reaction was completed, 232.8g of the product was obtained by vacuum distillation, that is, 3,4-dimethoxythiophene, the content was 98.5%, the total yield was 79.5%, and the ethylene glycol could be reused after recovery.
Embodiment 3
[0032] Add 552.3 g (2.0 mol) of sodium 2,5-dicarboxylate-3,4-thiophenediol to 1,400 g of N,N-dimethylformamide, stir at room temperature, and dropwise add 234.2 g of dimethyl carbonate ( 2.6mol), heated to reflux, and reacted for 3 hours; after the reaction was completed, evaporate the solvent to dryness and add 2200g (5.5mol) of sodium hydroxide solution with a mass concentration of 10% to mix evenly, heated to 82°C, reacted for 2 hours, and the reaction was completed Finally, acidify with sulfuric acid with a mass concentration of 50% at room temperature until the pH is 1 to 2, filter, add 1400 g of ethylene glycol solvent to the filter residue, add 63.8 g (0.4 mol) of copper sulfate under stirring, and heat to 110 ° C , heat preservation reaction for 5 hours, after the reaction was completed, 222.3g of the product, namely 3,4-dimethoxythiophene, was obtained by vacuum distillation, with a content of 98.2% and a total yield of 75.7%. The ethylene glycol can be reused after re...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com