Synthesis process of ribavirin intermediate and the intermediate
A technology of ribavirin and synthesis process, which is applied in the field of synthesis of ribavirin intermediates, can solve the problems of complicated synthesis technical route, difficult operation and high danger, and achieves simple and easy-to-obtain raw materials, simple operation and low production cost Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0075] A kind of synthetic technique of ribavirin intermediate 1,2,4-triazole-3-carboxamide, synthetic route (in structural formula A, B, C, R 1 =R 2 =-CH 3 ):
[0076]
[0077] Synthetic operation steps:
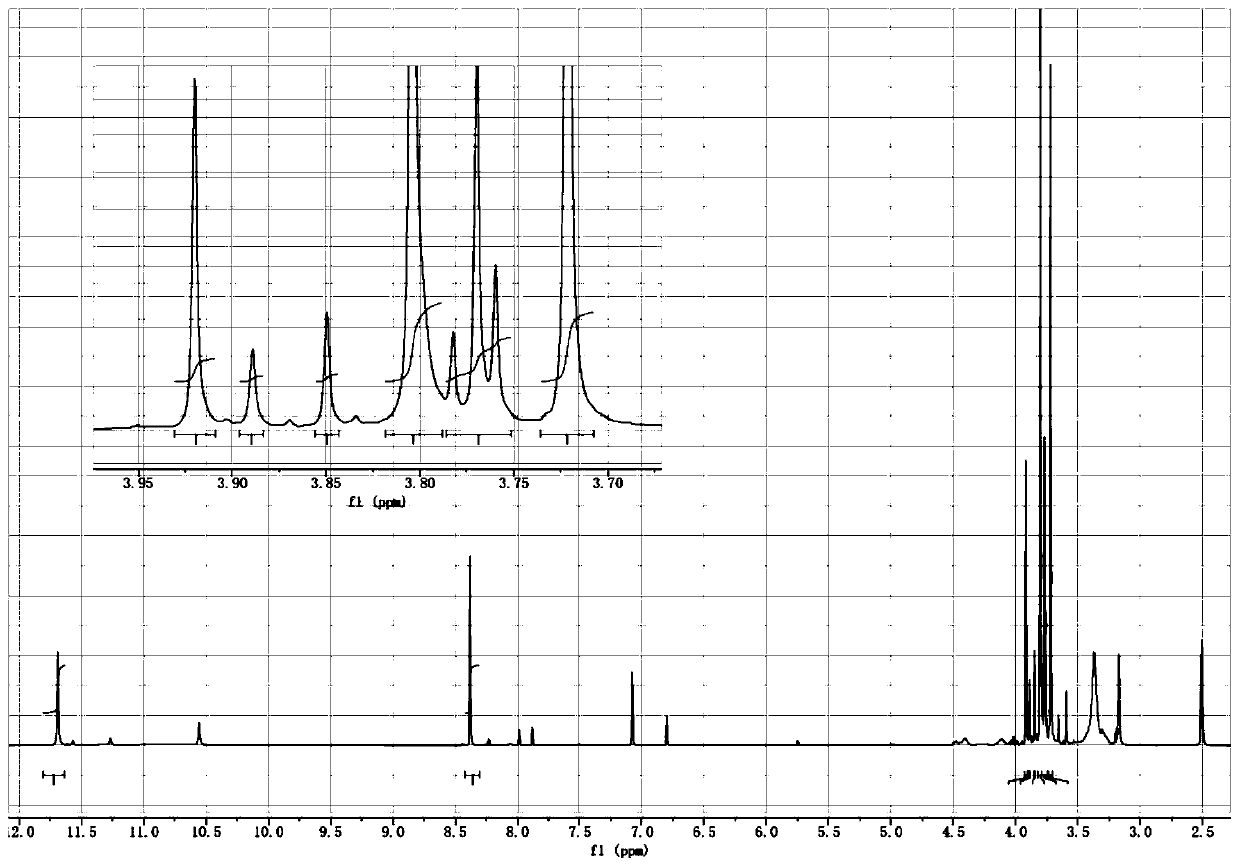
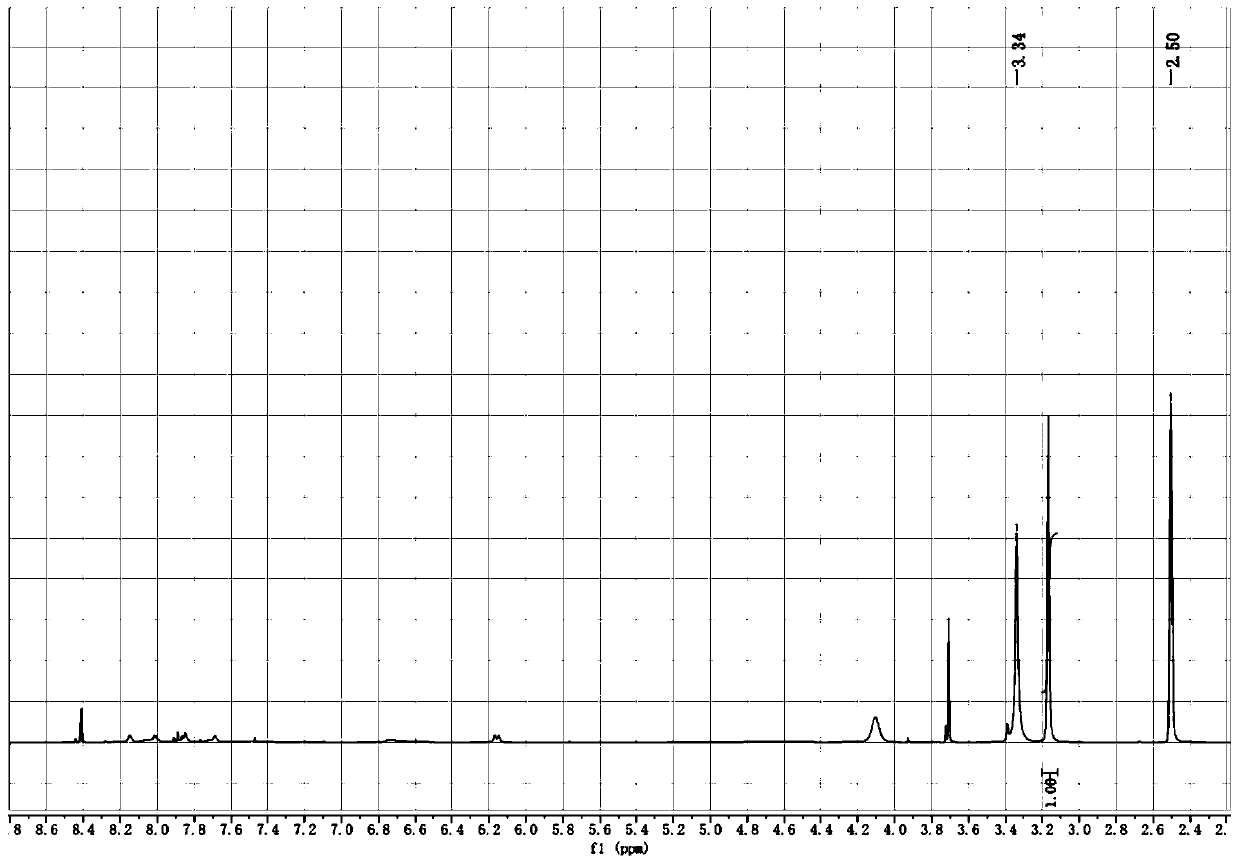
[0078] S1: 500mL three-necked bottle, equipped with magnetic stirring and thermometer; add 200mL of anhydrous methanol into it, add monohydrazide methyl oxalate (compound A) (59g, 0.5mol) into it under stirring, and then add trimethyl orthoformate in one go Ester (106.1g, 1.0mol), react at room temperature for 4h; after the reaction, concentrate and recover methanol and excess trimethyl orthoformate; the residue is oxalic acid monohydrazide methyl ether (oxalhydrazide methyl ether compound B, R 1 =R 2 =Me) 76g, theoretical yield: 95%. 1 H NMR (400MHz, DMSO-d 6 ) shows that the crude product is a mixture of four geometric isomers, which is consistent with the structure of two pairs of double bonds in the molecule. in, 1 HNMR (DMSO-d 6 ) spectrum (such as figure ...
Embodiment 5
[0081] According to the method of "Example 5", the product 1,2,4-triazole-3-carboxamide obtained in this step can be converted into 1,2,4-triazole-3-carboxylic acid methyl ester, proving that 1, The chemical structure of 2,4-triazole-3-carboxamide is correct.
Embodiment 2
[0083] This embodiment is based on embodiment 1, and the difference with embodiment 1 is:
[0084] What step S1 synthesized is oxalic acid monohydrazide ethyl ether B (R 1 =R 2 =Et), step S2 is starting raw material with oxalic acid monohydrazide ethyl ether, and the synthetic process of oxalic acid monohydrazide ethyl ether is as follows:
[0085] 500mL three-necked bottle equipped with magnetic stirring and thermometer, add 200mL of absolute ethanol, then add ethyl oxalic acid monohydrazide (0.5mol, R 1 =CH 3 CH 2 -), triethyl orthoformate (0.5mol), heated to reflux for 2h; after the reaction, concentrated. The residue is oxalic acid monohydrazide ethyl ether B (R 1 =R 2 =Et), yield 95%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com