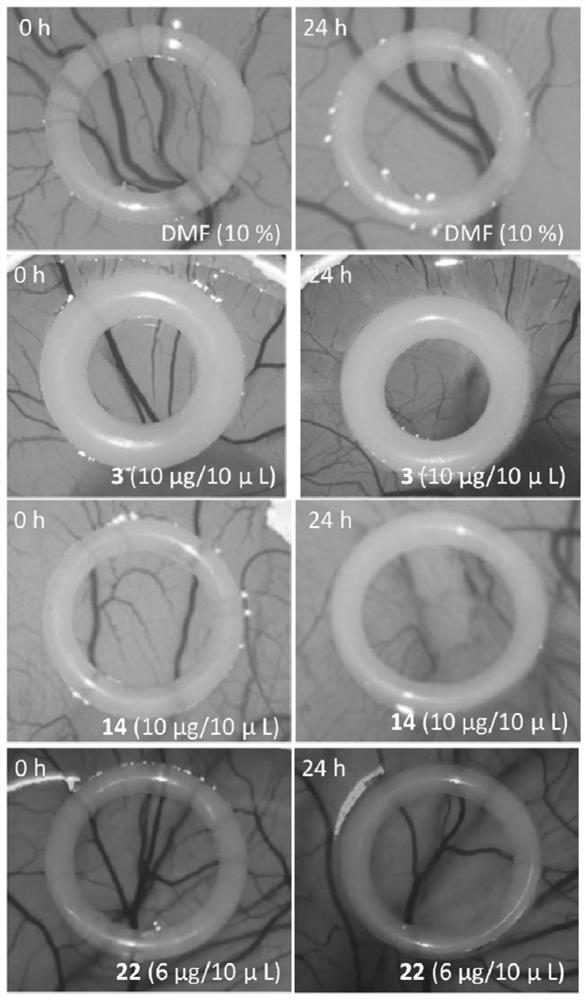
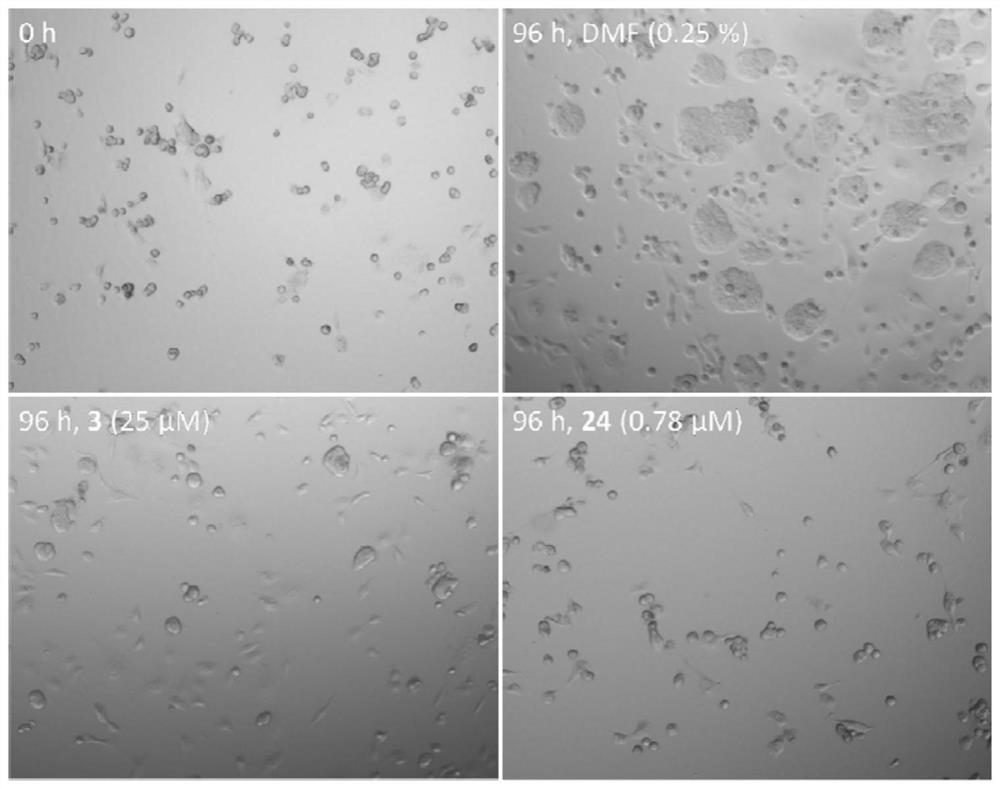
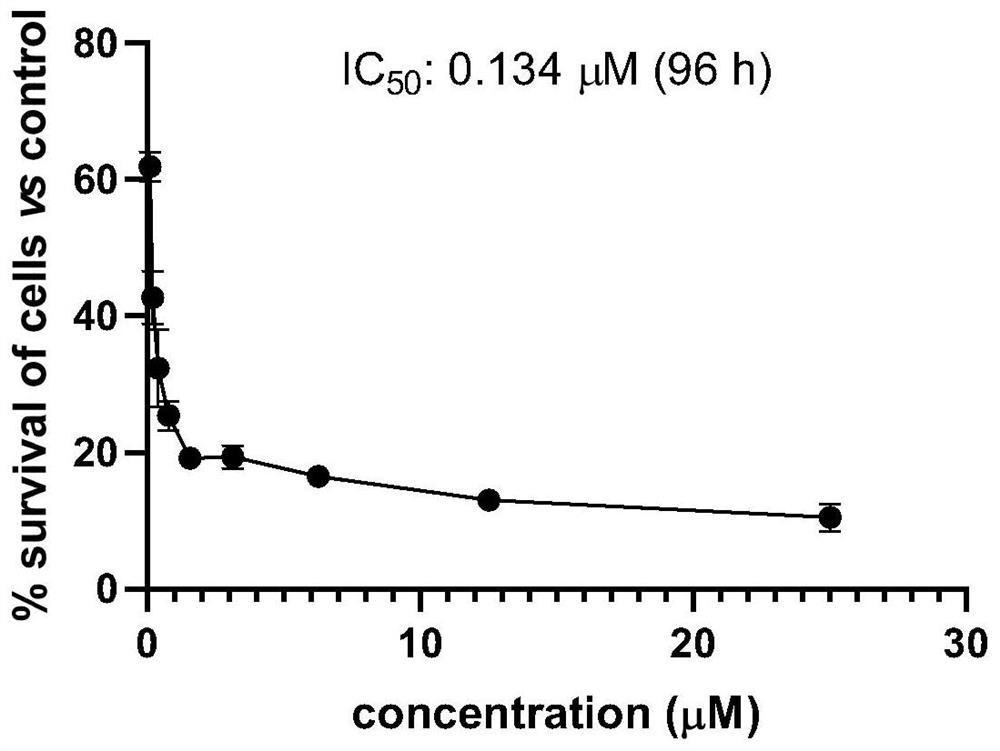
Metal complex containing tridentate ligand and xanthine derivative ligand and preparation method and medical application of metal complex
A technology of metal complexes and xanthines, which is applied in platinum-based organic compounds, medical preparations containing active ingredients, and compounds containing elements of group 1/11 of the periodic table, can solve toxic and side effects and limit the overall curative effect of combination therapy And other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] Synthesis of complex 1:
[0061]
[0062] Add compound 1,3-bis(2'-pyridyl) benzoplatinum chloride (II) (50mg, 0.11mmol) and CH in a 50mL three-necked flask 3 CN (8mL), add AgCF at reflux at 80°C 3 SO 3 (28mg, 0.11mmol), react in the dark for 1h and filter to remove insoluble matter. Add compound 1,3,7,9-tetramethylxanthine iodide (44mg, 0.13mmol) and CH in another 50mL three-necked flask 3 CN (5mL), dissolved completely under heating in 80℃ water bath, add AgCF 3 SO 3 (33mg, 0.13mmol), reacted at room temperature in the dark for 30min, then filtered to remove insoluble matter. The two filtrates were combined, t-BuOK (18mg, 0.16mmol) was added, and reacted at room temperature for 2.5h under the protection of nitrogen, then refluxed at 80°C for 18h. Cool to room temperature, filter to obtain a yellow clear filtrate, evaporate the solvent under reduced pressure, add 2 mL of CH to the crude product 3 Dissolve CN, add 6mL ether to form a precipitate, let the precip...
Embodiment 2
[0068] Synthesis of complex 2:
[0069]
[0070] With reference to the method of Example 1, 1,3,7,9-tetramethylxanthine iodide was replaced by 1-(2-hydroxyethyl)-3,7,9-trimethylxanthine iodide to obtain Complex 2, yield 14.4%.
[0071] 1 H-NMR (300MHz, DMSO-d 6 ): δ (ppm) 8.31 (d, J = 5.6Hz, 2H, ArH ),8.29-8.19(m,4H, ArH ),7.90(d,J=7.7Hz,2H, ArH ),7.44-7.31(m,3H, ArH ),4.90(t,J=5.8Hz,1H, Oh ),4.33(s,3H,N CH 3 ),4.14(s,3H,N CH 3 ),4.07(t,J=6.6Hz,2H,N CH 2 ),3.84(s,3H,N CH 3 ),3.60(q,J=6.4Hz,2H,HO CH 2 ).
[0072] Anal. Calcd for C 27 h 25 f 3 N 6 o 6 PtS: C 39.86, H 3.10, N 10.33. Found: C 39.28, H 3.10, N 10.38.
[0073] HR-MS(ESI)m / z[M] + Calcd for C 26 h 25 N 6 o 3 Pt, 664.1636; Found: 664.1631.
[0074] IR (cm -1 ):3442.94, 2966.52, 1701.22, 1664.57, 1539.20, 1257.59, 1159.22, 1029.99, 767.67, 636.51.
Embodiment 3
[0076] Synthesis of complex 3:
[0077]
[0078] With reference to the method of Example 1, 1,3,7,9-tetramethylxanthine iodide is replaced by 1-(5-oxohexyl)-3,7,9-trimethylxanthine iodide to obtain Complex 3, yield 19.7%.
[0079] 1 H-NMR (300MHz, CD 3 CN): δ(ppm)8.29-8.16(m,2H, ArH ),8.14(d,J=1.7Hz,1H, ArH ),8.12(d,J=1.6Hz,1H, ArH ),8.04-7.94(m,2H, ArH ),7.75(d,J=7.7Hz,2H, ArH ),7.41(t,J=7.7Hz,1H, ArH ),7.26(ddd,J=7.4,5.7,1.5Hz,2H, ArH ),4.33(s,3H,N CH 3 ),4.20(s,3H,N CH 3 ),4.03(t,J=6.9Hz,2H,N CH 2 ),3.83(s,3H,N CH 3 ), 2.55(t, J=6.7Hz, 2H, CO CH 2 ),2.12(s,3H,CO CH 3 ),1.72-1.55(m,4H,COCH 2 CH 2 CH 2 ).
[0080] Anal. Calcd for C 31 h 31 f 3 N 6 o 6 PtS: C 42.91, H 3.60, N 9.68. Found: C 42.96, H 3.38, N 9.81.
[0081] HR-MS(ESI)m / z[M] + Calcd for C 30 h 31 N 6 o 3 Pt, 718.2105; Found: 718.2104.
[0082] IR (cm -1 ):3460.30, 2935.66, 1707.00, 1662.64, 1541.12, 1261.45, 1145.72, 1031.92, 771.53, 636.51.
[0083] Wherein, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com