Method for continuously synthesizing 1H-1,2,3-triazole by using microchannel reactor
A microchannel reactor, 1H-1 technology, applied in chemical instruments and methods, chemistry/physics/physicochemical reactors, organic chemistry, etc., can solve problems such as potential safety hazards
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
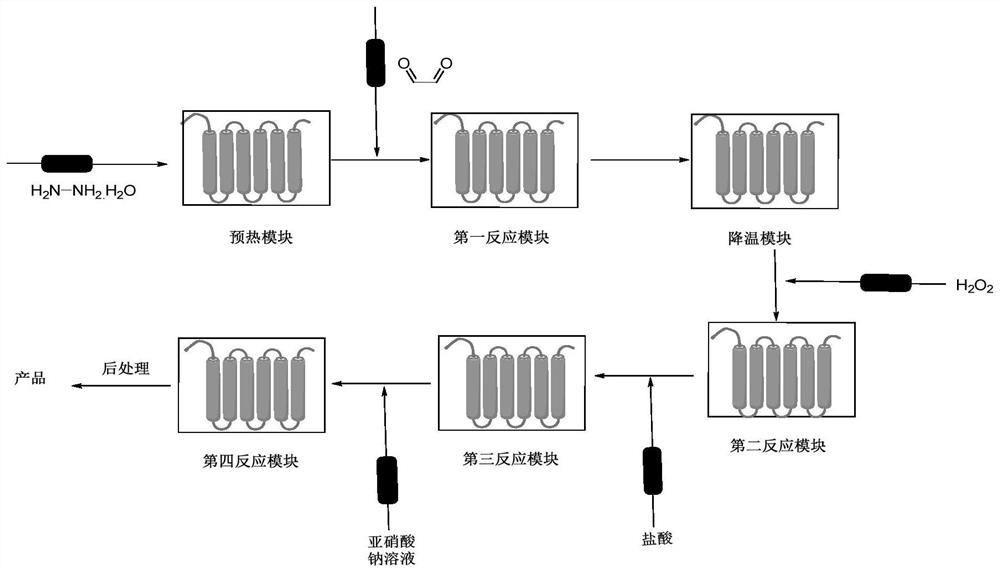
[0047]1) hydrazine hydrate aqueous solution (80%) 190g is delivered to preheating in the preheating module of microchannel reactor according to the flow rate of 40g / min, enters the first reaction module group of microchannel reactor after preheating, glyoxal Aqueous solution (40%) 200g is passed into the first reaction module by the flow velocity of 37.9g / min, and the first reaction module temperature is 100 ℃, and in the first reaction module, total residence time is 30s and hydrazine hydrate reacts, then in cooling The module was cooled to 30°C to obtain material 1;
[0048] 2) Material 1 continues to pass into the second reaction module, and at the same time, hydrogen peroxide solution (30%) is passed into the second reaction module at a flow rate of 32.6mL / min and undergoes oxidative cyclization reaction with material 1 at a reaction temperature of 30°C. The time is 30s, and the material 2 is obtained;
[0049] 3) Material 2 continues to pass into the third reaction modul...
Embodiment 2
[0053] 1) hydrazine hydrate aqueous solution (80%) 190g is delivered to preheating in the preheating module of microchannel reactor according to the flow rate of 40g / min, enters the first reaction module group of microchannel reactor after preheating, glyoxal Aqueous solution (40%) 200g is passed into the first reaction module by the flow rate of 40g / min, and the first reaction module temperature is 50 ℃, and in the first reaction module, total residence time is that 60s and hydrazine hydrate react, then in cooling module Cool down to 30°C to obtain material 1;
[0054] 2) Material 1 continues to pass into the second reaction module, and at the same time, hydrogen peroxide solution (30%) is passed into the second reaction module at a flow rate of 35mL / min to carry out oxidative cyclization reaction with material 1, the reaction temperature is 15°C, and the residence time Be 40s, make material 2;
[0055] 3) Material 2 continues to pass into the third reaction module of the mi...
Embodiment 3
[0059] 1) hydrazine hydrate aqueous solution (80%) 190g is delivered to preheating in the preheating module of microchannel reactor according to the flow rate of 40g / min, enters the first reaction module group of microchannel reactor after preheating, glyoxal Aqueous solution (40%) 200g passes into the first reaction module by the flow velocity of 35g / min, and the first reaction module temperature is 80 ℃, and in the first reaction module, total residence time is 42s and hydrazine hydrate reacts, then in cooling module Cool down to 35°C to obtain material 1;
[0060] 2) Material 1 continues to pass into the second reaction module, and at the same time, hydrogen peroxide solution (30%) is passed into the second reaction module at a flow rate of 39mL / min and undergoes oxidative cyclization reaction with material 1, the reaction temperature is 35 ° C, the residence time Be 30s, make material 2;
[0061] 3) material 2 continues to pass into the third reaction module of the microc...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com