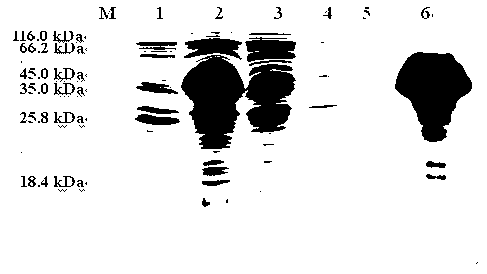Trichina recombinant protein and application thereof
A technology of recombinant protein and trichinella, which is applied in the field of recombinant protein and application of trichinella, and can solve the problems of unsatisfactory diagnosis and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
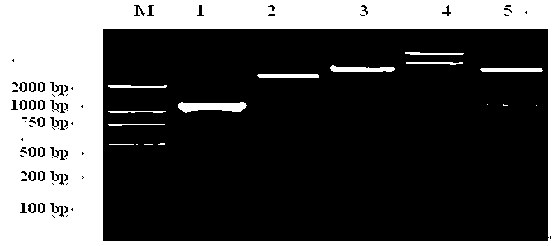
[0042] 1. Extraction of total RNA from Trichinella spiralis
[0043] Extracted with Trizol (invitrogen), the operation is as follows:
[0044] (1) The mice infected with Trichinella spiralis for 35 days were dissected, the skeletal muscles were taken and crushed, and the muscle larvae of Trichinella spiralis were recovered by artificial digestion. About 100 mg of fresh worms were taken, placed in a micro-homogenizer, and added 1mL of Trizol, sonicated quickly in an ice bath until no visible tissue pieces.
[0045] (2) Transfer the liquid to a DEPC-treated microcentrifuge tube and let stand at room temperature for 5 min; add 0.2 mL of phenol-chloroform extract, shake vigorously for about 15 s, and incubate at room temperature for 2–3 min.
[0046] (3) After centrifugation at 12,000×g at 4°C for 15 min, transfer the supernatant liquid to another clean centrifuge tube, add 200 μL of chloroform and extract again.
[0047] (4) Transfer the extracted upper layer liquid to a clean ...
Embodiment 2
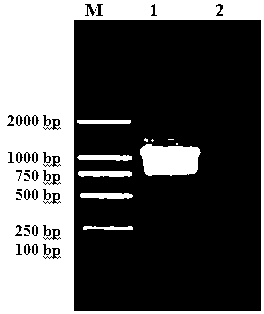
[0065] 1. Construction of prokaryotic expression pET30a-TsSerpin recombinant plasmid
[0066] pMD18-T-TsSerpin recombinant plasmid Eco R I and xho After I double enzyme digestion, cut the gel to recover the target gene fragment. The recovered target gene fragment and pET30a vector plasmid were respectively used Eco R I and xho I double enzyme digestion, the two enzyme digestion systems are: (1) 20 μL of the target gene fragment, 4 μL of 10× H buffer, Eco R I 2 μL, xho I 2 μL, BSA 2 μL, Triton 2 μL, sterilized double distilled water 8 μL; (2) vector plasmid 12 μL, 10× H buffer 4 μL, Eco R I 2 μL, xho I 2 μL, BSA 2 μL, Triton 2 μL, sterile double distilled water 16 μL. After the digestion mixture was centrifuged and mixed, it was digested in a water bath at 37°C for 4 h.
[0067] The digested product was recovered and purified with the gel recovery kit Agarose Gel DNA Extraction Kit, and then the product was ligated. The ligation system is: 7 μL of enzyme-digest...
Embodiment 3
[0072] 1. Purification of Recombinant Proteins
[0073] According to the method in Example 2, 1 L of bacterial liquid was co-induced, and the recombinant protein in the form of inclusion bodies was obtained by qualitative analysis. The inclusion body protein was purified by electroelution after gel cutting, and the protein content was measured by ultraviolet spectrophotometer. The formula is as follows:
[0074] Protein content (mg / mL)=(1.45×A 280 -0.74×A 260 )×dilution factor
[0075] The amount of recovered protein was determined to be about 6.58 mg of purified protein per 1 L of culture supernatant. After the purified TsSerpin recombinant protein was subjected to SDS-PAGE, there was only one obvious protein band at 48.5 kDa, and there were no other bacterial impurities, see Figure 4 .
[0076] 2. Western-blot identification of recombinant protein
[0077] The purified recombinant protein TsSerpin was first subjected to SDS-PAGE electrophoresis, and then transferred t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com