Novel slow-release breast perfusion agent for preventing cow subclinical mastitis and preparation method thereof
The technology of subclinical mastitis and perfusion agent is applied in the field of slow-release breast perfusion agent for preventing subclinical mastitis in dairy cows and its preparation field, which can solve the problems of hidden danger of food safety, economic loss, increase of white blood cell content and the like, and achieves reduction of drug administration frequency, good stability, and the effect of preventing recessive mastitis in dairy cows
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
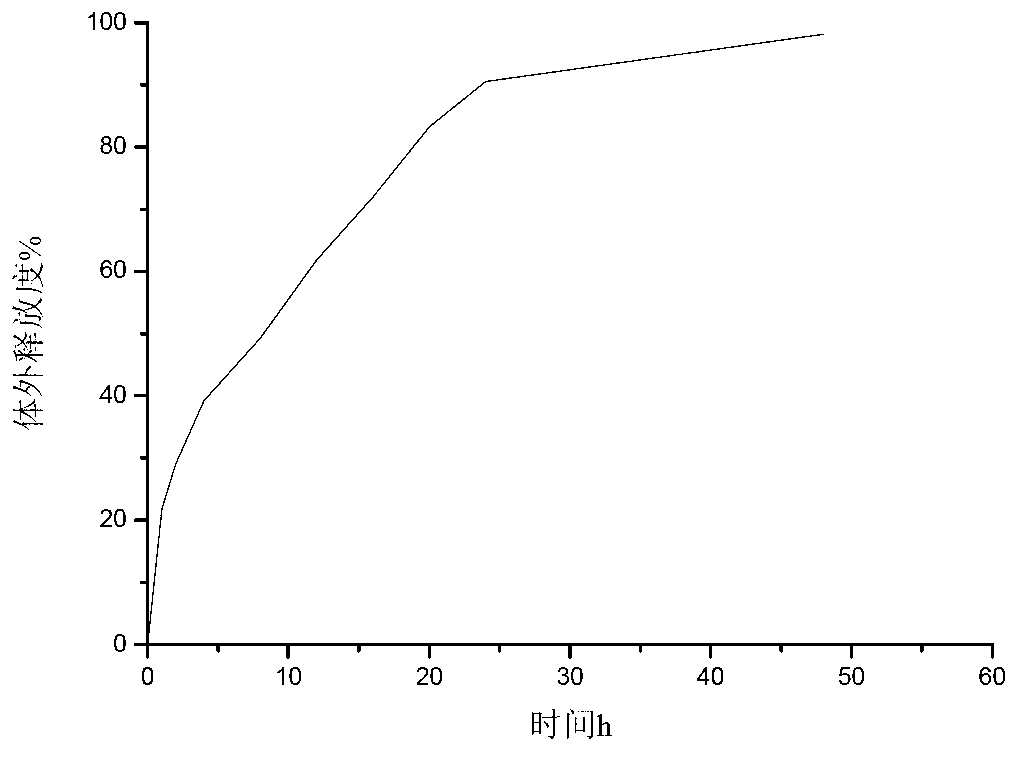
Image
Examples
Embodiment 1
[0015] Example 1 Sustained-release udder perfusion agent for preventing latent mastitis in dairy cows and its preparation
[0016] The composition of each 100g perfusion agent is as follows:
[0017]
[0018]
[0019] Crush benzathine cefapirin bulk drug until more than 90% of the particle diameter reaches 3-5 μm, and the sterilization meets the aseptic requirements, which is material A; sterilize the soybean oil for injection (sterilize by filtering with a sterile filter membrane), add Polyethylene glycol-400 (PEG-400), fatty alcohol polyoxyethylene ether (AEO-7), stir and mix, continue to heat for 5 hours, cool down to room temperature, it is liquid B; material A, liquid B, vitamin E , Benzyl alcohol is fed into a high-pressure homogenizer, and the high-pressure homogenizer is used to circulate and homogenize for 7 minutes. The pressure is controlled at 50Mpa, and the quantification is up to 100g. Packing, sealing, and packaging.
Embodiment 2
[0020] Example 2 Sustained-release udder perfusion agent for preventing latent mastitis in dairy cows and its preparation
[0021] The composition of each 100g perfusion agent is as follows:
[0022]
[0023] Crush benzathine cefapirin raw material until more than 95% of the particle diameter reaches 3-5 μm, and the sterilization meets the aseptic requirements, which is material A; sterilize the soybean oil for injection (sterilize by filtering with a sterile filter membrane), add Polyethylene glycol-400 (PEG-400), fatty alcohol polyoxyethylene ether (AEO-7), stir and mix, continue to heat for 4 hours, cool down to room temperature, and it is liquid B; material A, liquid B, vitamin E , Benzyl alcohol is fed into a high-pressure homogenizer, and the high-pressure homogenizer is used to circulate and homogenize for 5 minutes, the pressure is controlled at 150Mpa, and the quantification is up to 100g, and then packed, sealed, and packaged.
Embodiment 3
[0024] Example 3 Sustained-release udder perfusion agent for preventing latent mastitis in dairy cows and its preparation
[0025] The composition of each 100g perfusion agent is as follows:
[0026]
[0027]
[0028] Grind benzathine cefapirin bulk drug until more than 90% of the particle diameter reaches 3-5 μm, and sterilize to meet the sterile requirements, which is material A; sterilize the liquid paraffin (sterilize by filtering with a sterile filter membrane), add Tween -80, cyclomethylene glyceryl ether, stir and mix, continue to heat for 5 hours, drop to room temperature, and become liquid B; feed material A, liquid B, vitamin E, and benzyl alcohol into a high-pressure homogenizer, and use high-pressure homogenization The machine circulates homogeneously for 10 minutes, the pressure is controlled at 100Mpa, the quantitative is 100g, subpackaged, sealed and packaged.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com