Ratio-dependent florescent probe for identifying Zn<2+> and S<2-> in relayed manner as well as synthesis method and application of ratio-dependent florescent probe
A technology of fluorescent probes and synthesis methods, applied in chemical instruments and methods, fluorescence/phosphorescence, luminescent materials, etc., can solve the problems of inaccurate detection data, harmful environment, interference of measurement results, etc., and achieve simple product separation and purification process, Accurately detect and avoid the effect of external factors
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] (1) Synthetic relay recognition Zn 2+ and S 2- The reaction formula of the ratiometric fluorescent probe:
[0042]
[0043] (2) Synthetic relay recognition of Zn 2+ and S 2- The specific steps of the ratiometric fluorescent probe:
[0044] Weigh 300mg of the intermediate N -(2-(1H-Benzimidazolyl)phenyl)-2-chloroacetamide (compound 2) and 418mg of raw material bis-(pyridylmethylene)amine (compound 3), dissolved in 20ml of N,N - In dimethylformamide (DMF), stir the reaction for 7 hours at room temperature, add 100ml of distilled water, adjust the pH value to 6 with dilute hydrochloric acid, collect the resulting precipitate by filtration, and recrystallize with acetonitrile to obtain a fluorescent probe N -(2-(1H-Benzimidazolyl)phenyl)-2-bis(2-pyridinemethylamino)acetamide (Compound 1), the yield was 56%.
Embodiment 2
[0046] Weigh 300mg of the intermediate N -(2-(1H-Benzimidazolyl)phenyl)-2-chloroacetamide and 630mg of bis-(pyridylmethylene)amine were dissolved in 20ml of DMF, stirred and reacted for 9h at room temperature, Add 150ml of distilled water, adjust the pH to 7 with dilute hydrochloric acid, collect the resulting precipitate by filtration, and recrystallize with acetonitrile to obtain a fluorescent probe N -(2-(1H-Benzimidazolyl)phenyl)-2-bis(2-pyridinemethylamino)acetamide, the yield is 68%.
Embodiment 3
[0048] Weigh 300mg of the intermediate N -(2-(1H-Benzimidazolyl)phenyl)-2-chloroacetamide and 835mg of bis-(pyridylmethylene)amine were dissolved in 20ml of DMF, and the reaction was stirred at room temperature for 10h, Add 150ml of distilled water, adjust the pH to 7 with dilute hydrochloric acid, collect the resulting precipitate by filtration, and recrystallize with acetonitrile to obtain a fluorescent probe N -(2-(1H-Benzimidazolyl)phenyl)-2-bis(2-pyridinemethylamino)acetamide, the yield was 72%.
[0049] The fluorescent probe of embodiment 1~embodiment 3 N Basic data for -(2-(1H-benzoimidazolyl)phenyl)-2-bis(2-pyridinemethylamino)acetamide:
[0050] Melting point: 177℃~179℃;
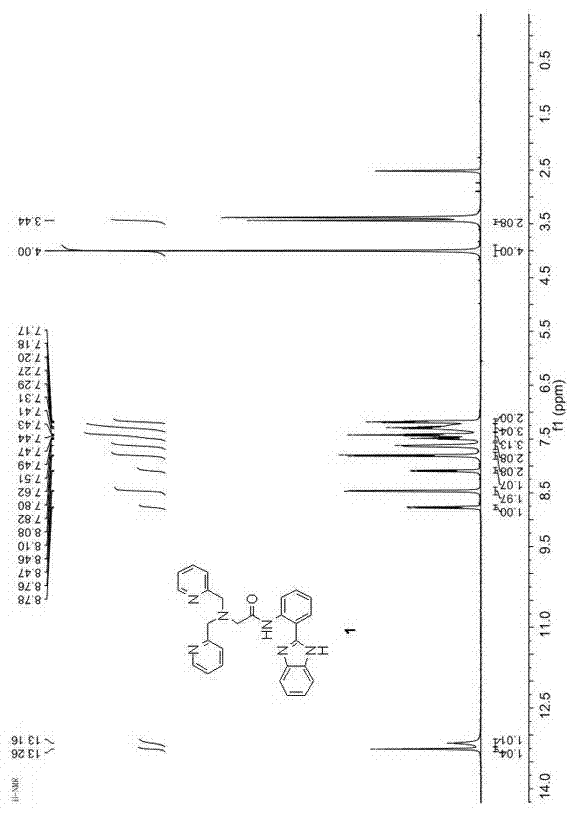
[0051] 1 H NMR (400 MHz, DMSO- d 6 ) δ 13.20 (s, 1H), 13.09 (s, 1H), 8.72 (d, J = 8.4 Hz, 1H), 8.41 (d, J = 4.3 Hz, 2H), 8.04 (d, J = 7.8 Hz, 1H), 7.75 (d, J = 7.8 Hz, 2H), 7.57 (s, 2H), 7.47-7.31 (m, 3H), 7.32-7.04 (m, 5H), 4.13-3.84 (m, 4H), 3.39 (S, 2H). (eg figure 1 ).
[0052...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com