Preparation method of monoamino-terminated polyethylene glycol-polyester amphiphilic block copolymer and obtained product
An amphiphilic block, polyethylene glycol technology, applied in the field of chemical synthesis, can solve the problem of difficult control of the ratio of polyethylene glycol and poly-e-caprolactone, the decrease of polymer molecular weight controllability, temperature conditions Large changes and other issues, to achieve the effect of easy implementation, mild conditions, and easy conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Preparation of Monoamino Polyethylene Glycol
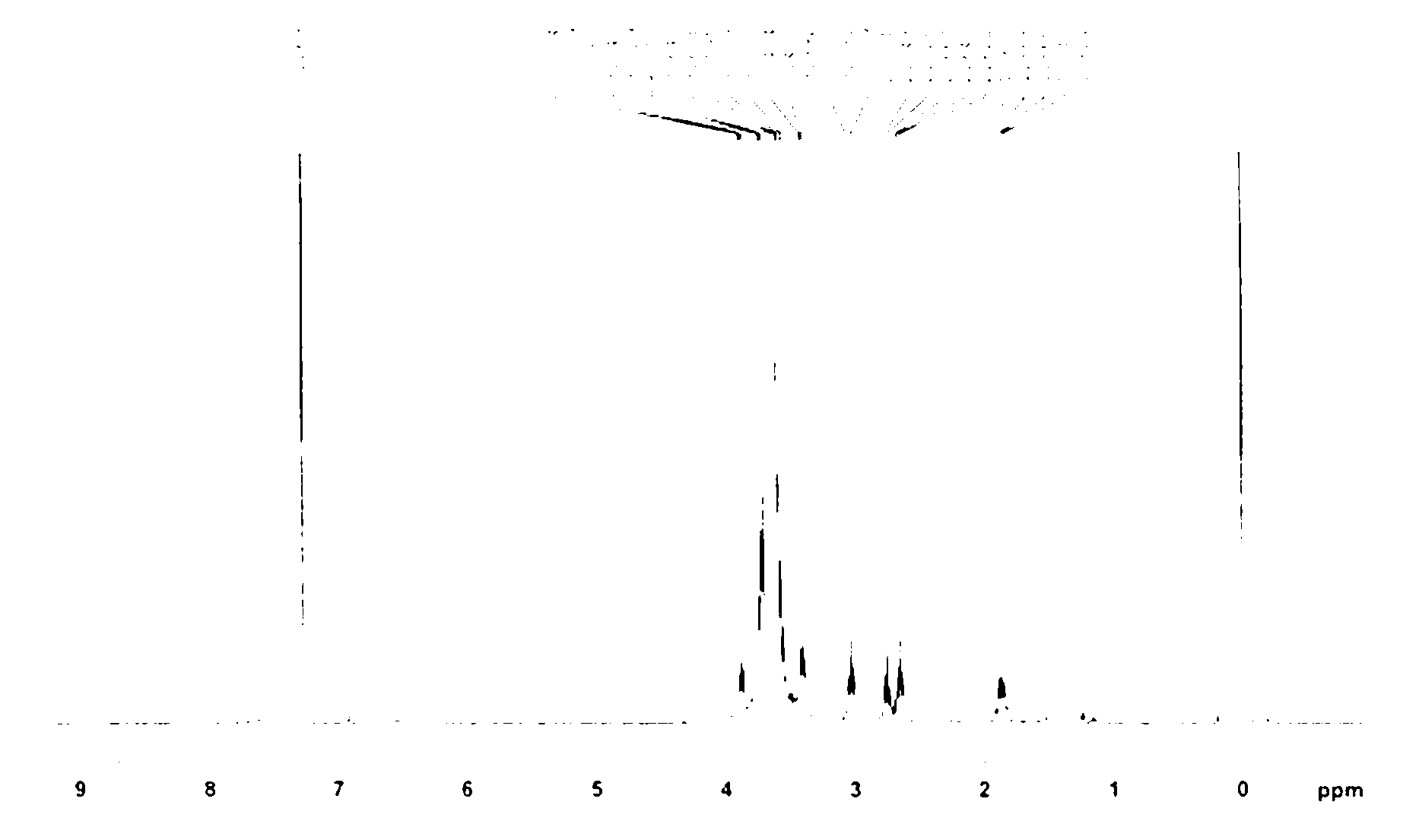
[0041] In a dry reaction flask, add N, N-dimethylformamide (20 ml), then add polyethylene glycol-2400 monoallyl ether (2.4 g, 1 mmol), mercaptoethylamine hydrochloride ( 2.27 g, 20 mmol) and azobisisobutyronitrile (3.17 g, 12 mmol), heated to 60-65°C, and reacted for 24 h. Remove most of the solvent by distillation under reduced pressure, add 10 ml of distilled water, control the temperature at 25-30 °C, add 1 mol / L potassium carbonate aqueous solution (20 ml) dropwise, evaporate the water under reduced pressure, cool down to room temperature, add 30 ml of dichloro Methane, stirred for 0.5 h, filtered, and the filtrate was dried overnight with anhydrous sodium sulfate, evaporated under reduced pressure until about 3 ml of solvent remained, added 30 ml of anhydrous ether to precipitate, and filtered with suction. The resulting white solid was dissolved in as little dichloromethane as possible, and precipitated with 10 times...
Embodiment 2
[0047] Preparation of Monoamino Polyethylene Glycol
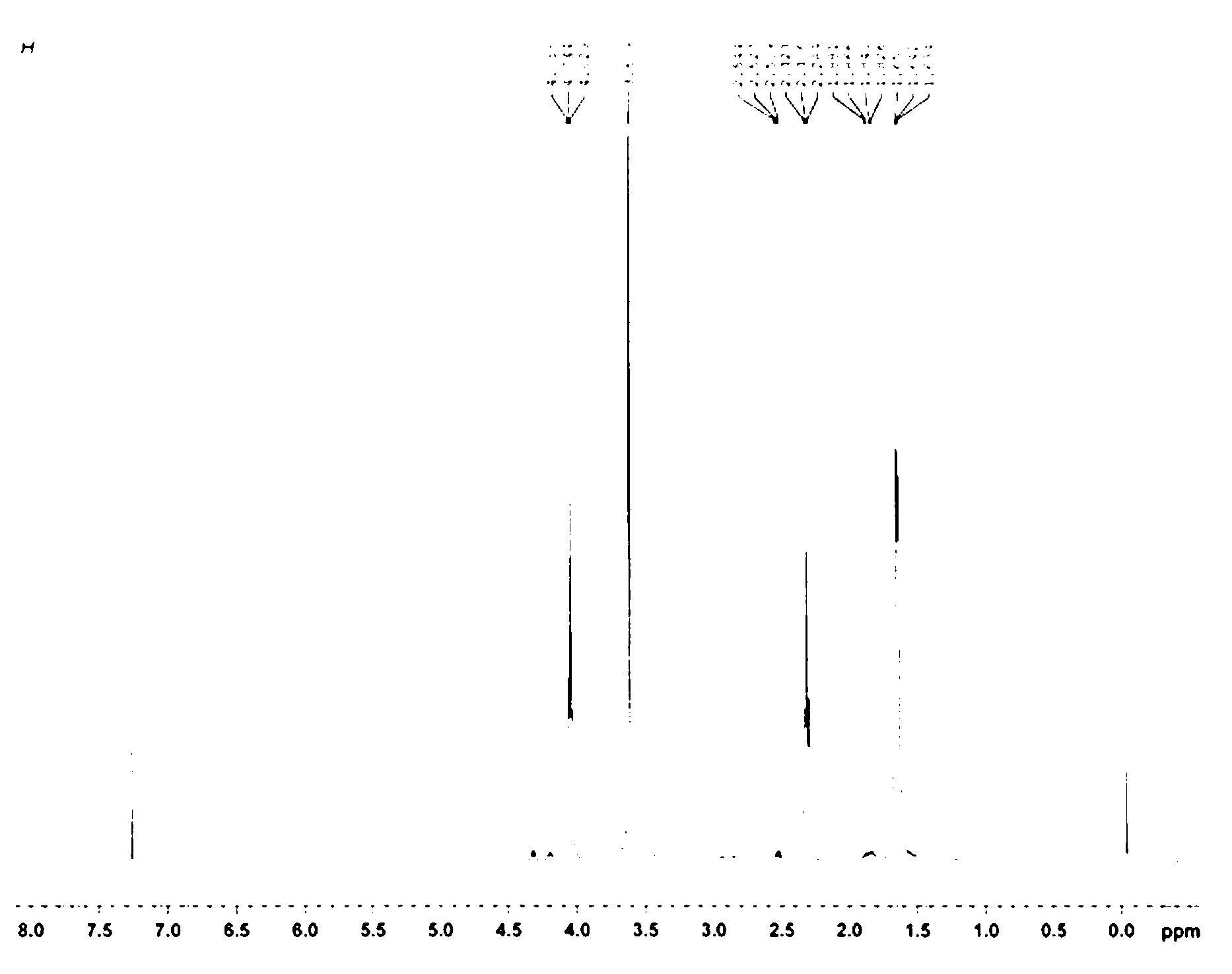
[0048] In a dry reaction flask, add 1,4-dioxane (20 ml), then add polyethylene glycol-2400 monoallyl ether (2.4 g, 1 mmol), mercaptoethylamine hydrochloride (1.14 g, 10 mmol) and azobisisobutyronitrile (0.264 g, 1 mmol), heated to 60-65°C, and reacted for 24 h. Remove most of the solvent by distillation under reduced pressure, add 10 ml of distilled water, control the temperature at 25-30 °C, add dropwise 1 mol / L potassium carbonate aqueous solution (20 ml), evaporate the water under reduced pressure, cool down to room temperature, add 30 ml of dichloro Methane, stirred for 0.5 h, filtered, and the filtrate was dried overnight with anhydrous sodium sulfate, evaporated under reduced pressure until about 3 ml of solvent remained, added 30 ml of anhydrous ether to precipitate, and filtered with suction. The resulting white solid was dissolved in as little dichloromethane as possible, and precipitated with 10 times the amount ...
Embodiment 3
[0050] Preparation of monoaminopolyethylene glycol-poly-d-valerolactone
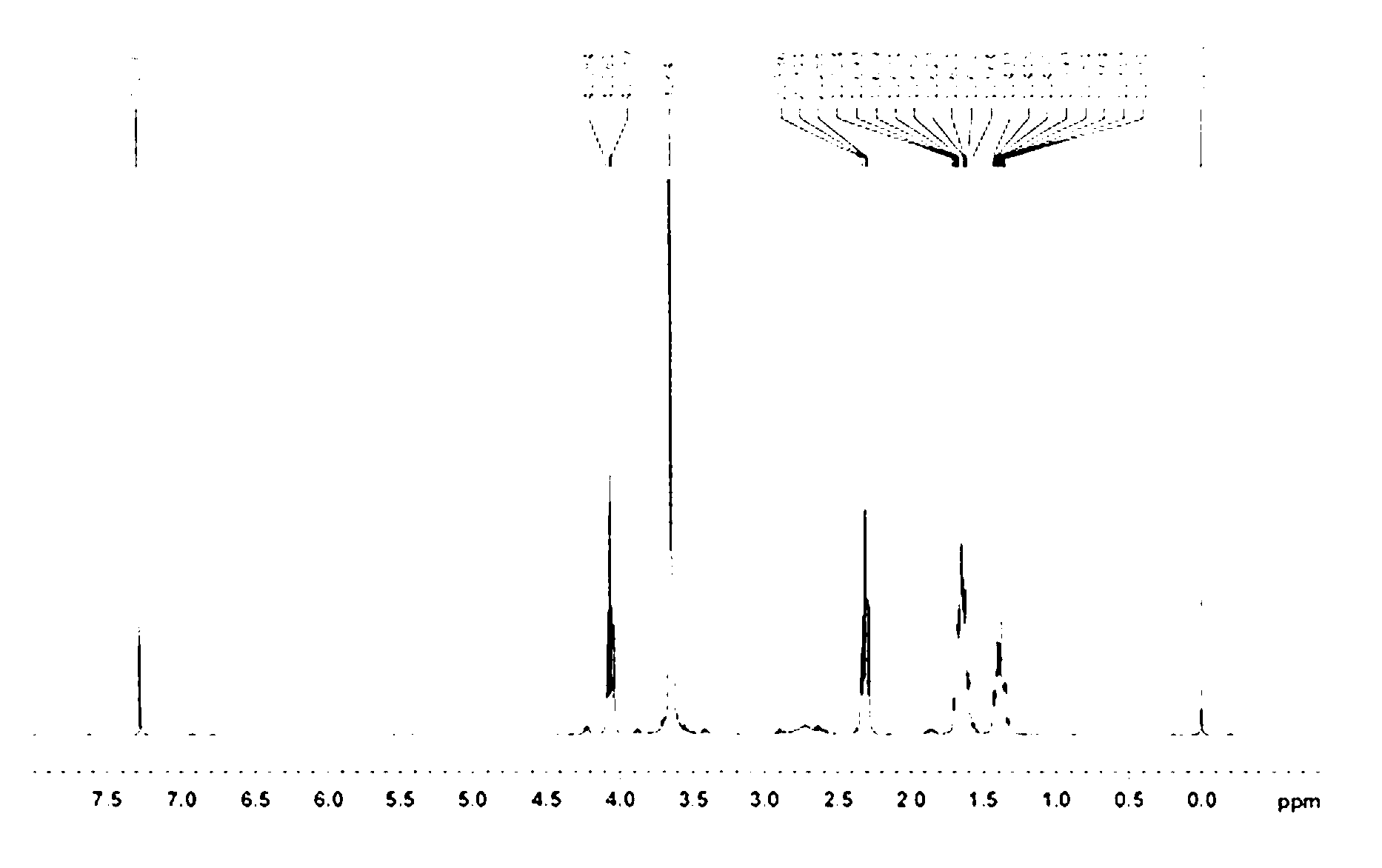
[0051] In a dry reaction flask, mix and dissolve the monoaminopolyethylene glycol-2400 (2.48 g, 1 mmol) obtained in Example 1, 1 mol / L diethyl ether hydrochloride (2 ml) and chloroform (100 ml), then add d-valerolactone (4.96 g, 49.6 mmol) was reacted at room temperature for 24 h, washed three times with saturated aqueous sodium carbonate solution, dried over anhydrous sodium sulfate, and the organic solvent was distilled off under reduced pressure. A small amount of dichloromethane was added to dissolve the resulting glassy solid, and it was precipitated with 10 times the amount of anhydrous ether. Repeated twice to obtain 6.55 g of powdery white solid with a yield of 89%, and the number of repeating units of polyester in the obtained polymer was 57. 1 H-NMR spectrum such as image 3 . Explanation: This compound is the target compound, poly d-valerolactone is introduced, and the polyester structure i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com