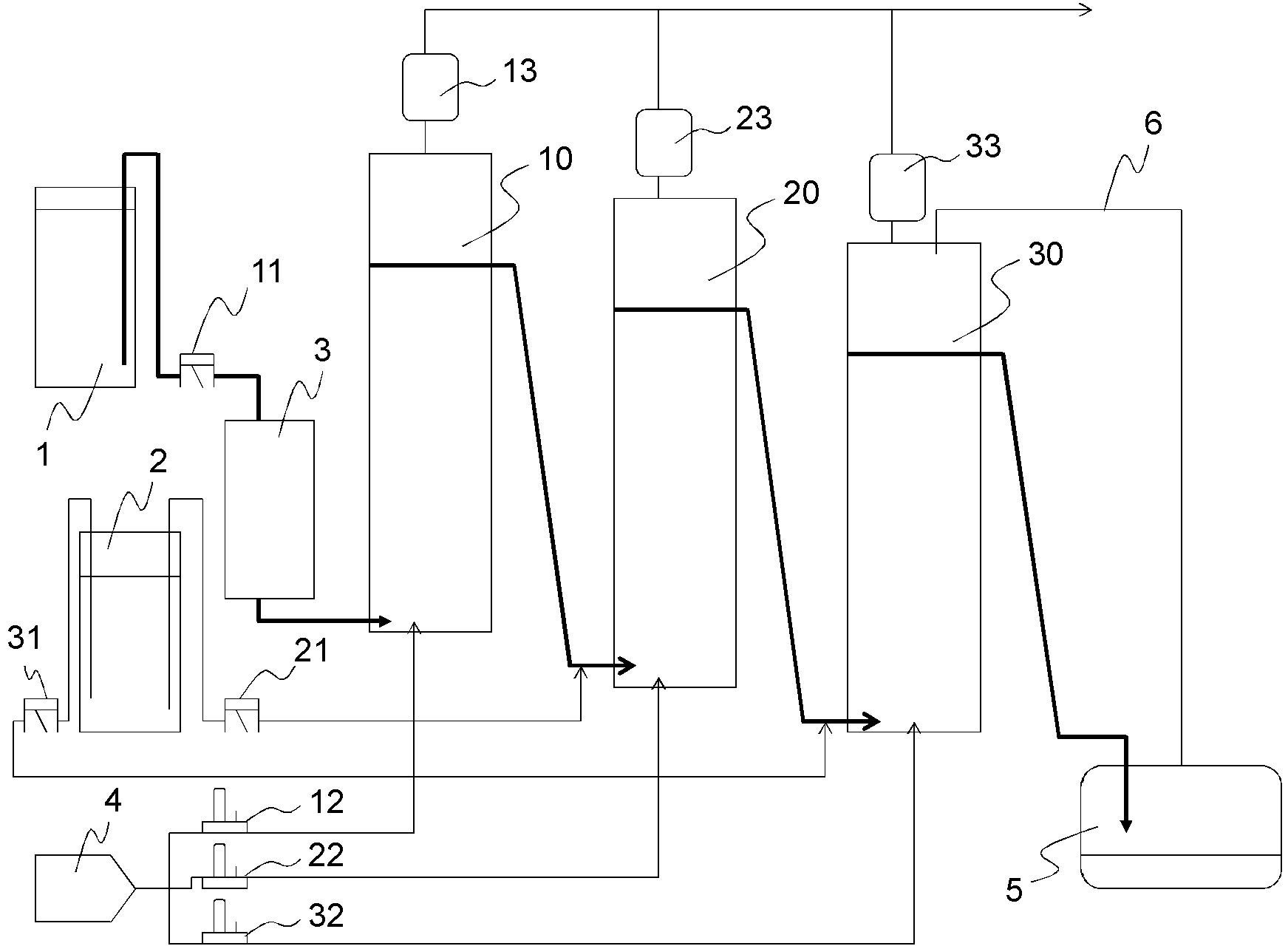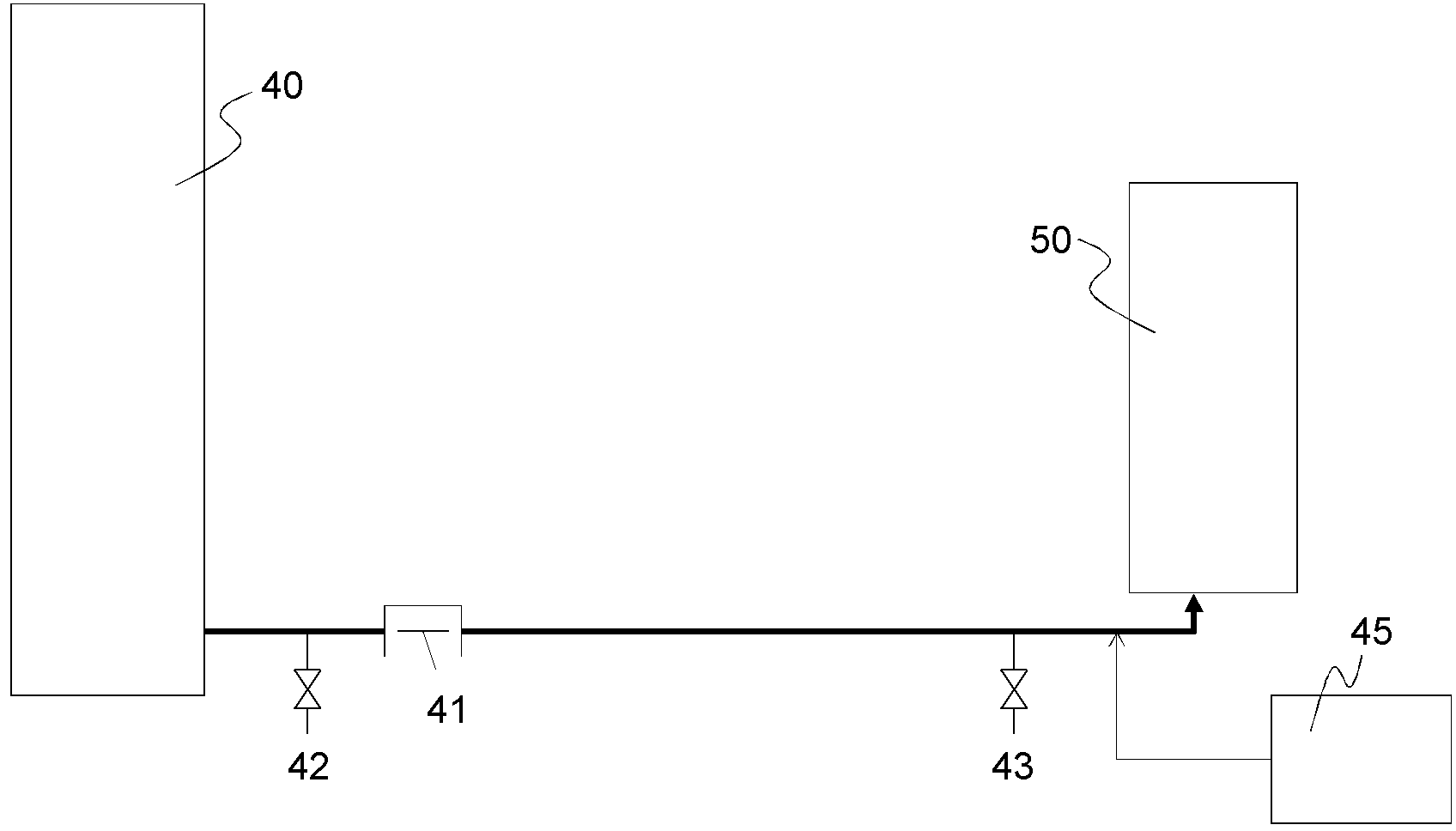Oxidation catalyst for hydrocarbon compound, and method and apparatus for producing oxide of hydrocarbon compound using same
A technology for oxidation catalysts and hydrocarbon compounds, which is applied in the directions of organic compound/hydride/coordination complex catalysts, preparation of organic compounds, preparation of peroxy compounds, etc., and can solve the problem of reducing the yield of cyclohexanone and cyclohexanol and accelerating the Hydrocarbon compound oxidation reaction, insufficient selectivity, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
no. 1 approach
[0061] The easiness of oxidation of a hydrocarbon compound by molecular oxygen is affected by the bond energy between skeleton carbon and hydrogen. Generally, the bond energy of carbon-hydrogen increases in the order of tertiary carbon, secondary carbon, and primary carbon. In addition, the electron-withdrawing substituent, the aromatic substituent, and the carbon-carbon double bond have the effect of reducing the carbon-hydrogen bond energy of the carbon to which the substituent and the like are bonded and the adjacent carbon. Therefore, straight-chain hydrocarbons and cyclic hydrocarbons having no substituents are generally less likely to be oxidized.
[0062] For example, cyclohexane has a carbon-hydrogen bond energy as high as 94 kcal / mol (Non-Patent Document 1), and is difficult to be oxidized. This is why cyclohexanone and cyclohexanol cannot be obtained in high yields even though cyclohexane is an industrially important compound.
[0063] In order to promote the oxida...
no. 2 approach
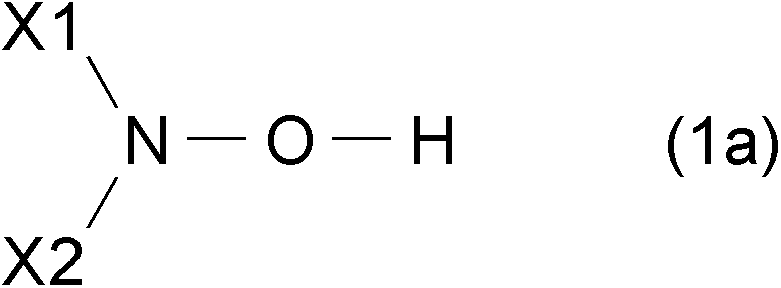
[0135] The oxidation catalyst according to the second embodiment of the present invention is an oxidation catalyst comprising the N-hydroxy compound represented by the above-mentioned general formula (1a) and general formula (1b), more preferably comprising the following general formula (3) oxidation catalysts of oxime compounds. The oxime compound represented by general formula (3) can be used individually by 1 type, or can use it in combination of 2 or more types. The oxidation catalyst according to the second embodiment of the present invention has a large effect of promoting the oxidation reaction of hydrocarbon compounds, and can oxidize hydrocarbon compounds even if it is used in a small amount.
[0136]
[0137] In the general formula (3), R1 and R2 are independently substituted or unsubstituted alkyl, substituted or unsubstituted alkenyl, substituted or unsubstituted alkynyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted A substituted cycloa...
no. 3 approach
[0173] In a third embodiment of the present invention, a hydrocarbon compound is oxidized by means of molecular oxygen (commonly referred to as "auto-oxidation"), thereby forming the corresponding hydroperoxide. Furthermore, the hydroperoxide is decomposed to produce the corresponding ketone and / or alcohol.
[0174] Here, ketones and alcohols are more easily oxidized than hydrocarbon compounds as starting materials, and oxides in high oxidation states such as carboxylic acids are by-produced. Therefore, the following methods are generally used: a method of performing an oxidation reaction without adding a transition metal compound such as cobalt, which is generally used as an oxidation catalyst (called a catalyst-free oxidation method); The method of suppressing the decomposition of hydroperoxide in the oxidation step by using a chemical agent (Patent Document 1), and the method of decomposing the hydroperoxide in a non-oxidizing atmosphere in the next step (referred to as a h...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com